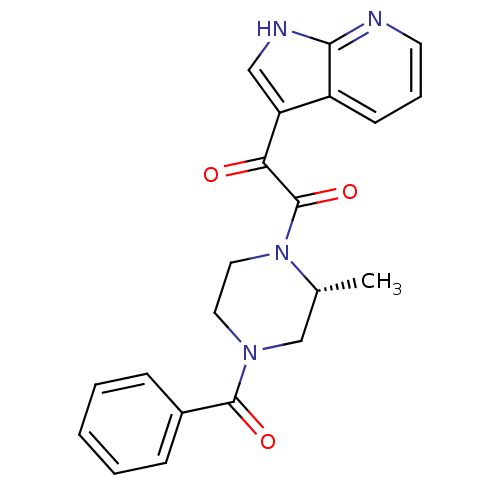
BDBM50228518 (R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyrrolo[2,3-b]pyridin-3-yl)ethane-1,2-dione::CHEMBL442078
SMILES C[C@@H]1CN(CCN1C(=O)C(=O)c1c[nH]c2ncccc12)C(=O)c1ccccc1
InChI Key InChIKey=CCGWWGUXCYXFJW-UHFFFAOYSA-N
Data 7 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50228518
Found 7 hits for monomerid = 50228518
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibition of recombinant CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of recombinant CYP3A4 using 7-benzyloxyresorufin as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of recombinant CYP3A4 using benzyloxy-4-(trifluoromethyl)coumarin as substrateMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 8.00E+4nMAssay Description:Inhibition of human ERG by channel flux assayMore data for this Ligand-Target Pair