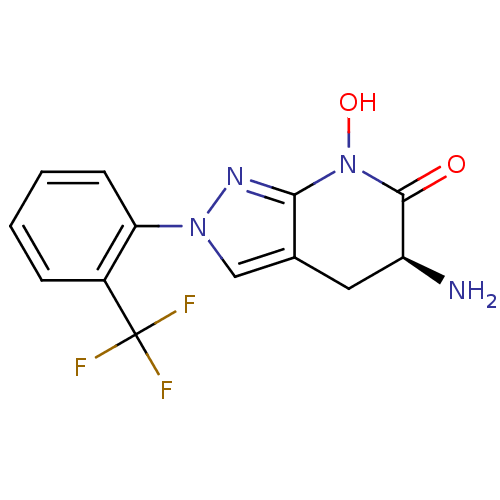
BDBM107744 US8933095, 28
SMILES N[C@H]1Cc2cn(nc2N(O)C1=O)-c1ccccc1C(F)(F)F
InChI Key InChIKey=DIOXJIHGGZCWPB-VIFPVBQESA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 107744
Found 2 hits for monomerid = 107744
TargetKynurenine/alpha-aminoadipate aminotransferase, mitochondrial(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 38.8nMT: 2°CAssay Description:Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ...More data for this Ligand-Target Pair
TargetKynurenine/alpha-aminoadipate aminotransferase, mitochondrial(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 38.8nMAssay Description:Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ...More data for this Ligand-Target Pair