BDBM151611 SP-2509::US11433053, Example HCI-2509::US8987335, 12::US9555024, 12
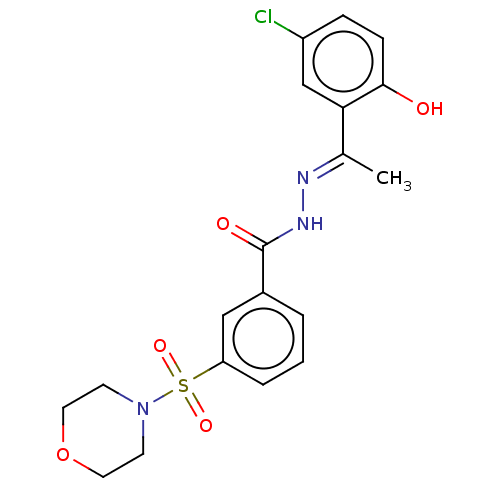
SMILES C\C(=N/NC(=O)c1cccc(c1)S(=O)(=O)N1CCOCC1)c1cc(Cl)ccc1O
InChI Key InChIKey=NKUDGJUBIVEDTF-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 151611
Found 8 hits for monomerid = 151611
Affinity DataIC50: 11nMpH: 7.5 T: 2°CAssay Description:The LSD1 screening biochemical assay was performed by Shanghai ChemPartner Co. Ltd and the detailed protocol was shown as followed. The AlphaLISA ass...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:The purpose of this test is to test the in vitro inhibitory activities of the compounds against LSD1. The enzyme used in this experiment was human LS...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of monoamine oxidase activity was carried used using the MAO-GIo Assay Kit according to the manufacturer's suggested protocol. Briefly...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of monoamine oxidase activity was carried used using the MAO-GIo Assay Kit according to the manufacturer's suggested protocol. Briefly...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of monoamine oxidase activity was carried used using the MAO-Glo.TM. Assay Kit according to the manufacturer's suggested protocol. Bri...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of monoamine oxidase activity was carried used using the MAO-Glo.TM. Assay Kit according to the manufacturer's suggested protocol. Bri...More data for this Ligand-Target Pair