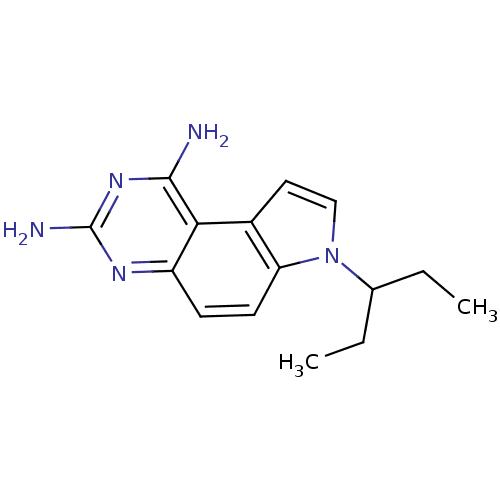
BDBM18043 1,3-DIAMINO-7-(1-ETHYEPROPYE)-7H-PYRRALO-[3,2-F]QUINAZOLINE::5-(pentan-3-yl)-5,10,12-triazatricyclo[7.4.0.0^{2,6}]trideca-1(13),2(6),3,7,9,11-hexaene-11,13-diamine::CHEMBL318721::GW345
SMILES CCC(CC)n1ccc2c1ccc3c2c(nc(n3)N)N
InChI Key InChIKey=GCPJCLJGTVTGRF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 18043
Found 4 hits for monomerid = 18043
Affinity DataKi: 0.220nMAssay Description:Inhibition of Dihydrofolate reductase enzyme from Candida albicansMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 6.4 T: 2°CAssay Description:IC50 is the concentration of inhibitor that decreases the velocity of the standard assay by 50%. The enzyme, NADPH, and varying concentrations of inh...More data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Inhibition of human recombinant Dihydrofolate reductase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60nMpH: 7.0 T: 2°CAssay Description:IC50 is the concentration of inhibitor that decreases the velocity of the standard assay by 50%. The enzyme, NADPH, and varying concentrations of inh...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)