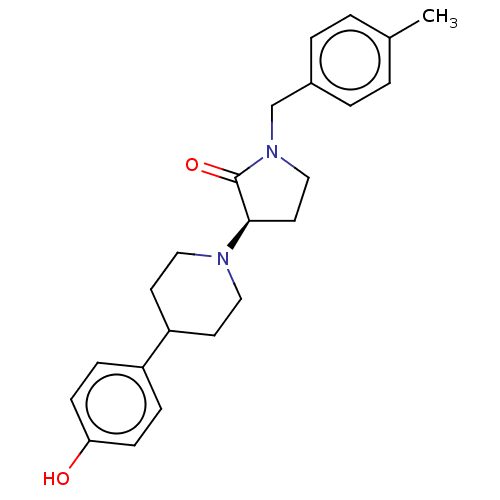
BDBM198694 US9221796, 23b
SMILES Cc1ccc(CN2CC[C@@H](N3CCC(CC3)c3ccc(O)cc3)C2=O)cc1
InChI Key InChIKey=ILUHFPBBZNEUNW-JOCHJYFZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 198694
Found 3 hits for monomerid = 198694
Affinity DataKi: 1.40nM ΔG°: -13.1kcal/moleT: 2°CAssay Description:To perform the competition binding assay, thawed membrane homogenate was
added to each well of a 96-well plate (20 ug/well). The experimental
compo...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology...More data for this Ligand-Target Pair