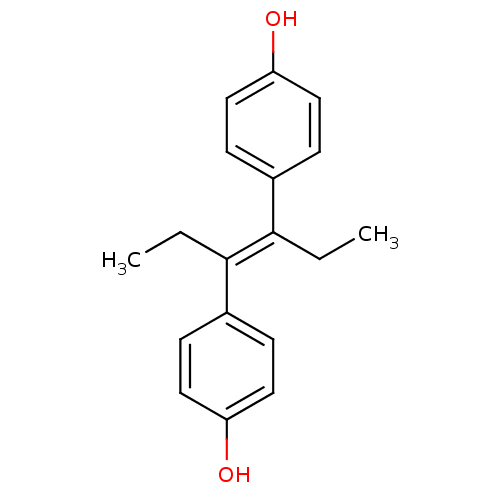
BDBM20625 4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol::4-[(E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol::CHEMBL411::Diethylstilbestrol::Diethylstilbestrol (1)::Stilbestrol::Stilboestroform
SMILES CC/C(=C(/CC)\c1ccc(cc1)O)/c2ccc(cc2)O
InChI Key InChIKey=RGLYKWWBQGJZGM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 27 hits for monomerid = 20625
Found 27 hits for monomerid = 20625
Affinity DataEC50: 0.00700nMAssay Description:In vitro agonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells at 10 pMMore data for this Ligand-Target Pair
Affinity DataIC50: 9nM EC50: 0.0200nMpH: 7.4 T: 2°CAssay Description:Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA...More data for this Ligand-Target Pair
Affinity DataIC50: 12nM EC50: 0.0600nMpH: 7.4 T: 2°CAssay Description:Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA...More data for this Ligand-Target Pair
Affinity DataEC50: 0.110nMAssay Description:Selective estrogen receptor down-regulator activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as induction of ERalph...More data for this Ligand-Target Pair
Affinity DataKi: 0.126nMAssay Description:Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol)More data for this Ligand-Target Pair
Affinity DataKi: 0.128nMAssay Description:Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanolMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]estradiol from human ER-alpha expressed in Sf21 cells incubated for 120 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as induction of ER-alpha-mediated transcriptional activit...More data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMAssay Description:Displacement of radioligand from Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Displacement of [3H]estradiol from human ER-alpha expressed in Sf21 cells incubated for 120 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 2.5nMAssay Description:Agonist activity at ER-alpha (unknown origin) by ER-alpha coactivator assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of [3H]17-beta-estradiol binding to human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In vitro inhibitory concentration against [3H]17-beta-estradiol binding to human estrogen receptor 2More data for this Ligand-Target Pair
Affinity DataIC50: 460nMAssay Description:Inhibition of human aldehyde oxidaseMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Human)
Japanese Foundation For Cancer Research
Curated by ChEMBL
Japanese Foundation For Cancer Research
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:TP_TRANSPORTER: drug resistance(SN-38) in BCRP-expressing K562 cellsMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Human)
Japanese Foundation For Cancer Research
Curated by ChEMBL
Japanese Foundation For Cancer Research
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:TP_TRANSPORTER: drug resistance(Mitoxantrone) in BCRP-expressing K562 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 610nMAssay Description:Displacement of [3H]-estradiol from human recombinant ERbeta expressed in Sf21 cells after 2 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 630nMpH: 7.0 T: 2°CAssay Description:Recruitment of the RIP140 NR-box peptide to the ERRgamma ligand binding domain was assayed by FRET detection. Proteins were set up in a one to one ra...More data for this Ligand-Target Pair
Affinity DataIC50: 770nMAssay Description:Displacement of [3H]-estradiol from human recombinant ERalpha expressed in Sf21 cells after 2 hrsMore data for this Ligand-Target Pair
TargetSarcoplasmic/endoplasmic reticulum calcium ATPase 1(Rabbit)
University of Chemistry and Technology Prague
Curated by ChEMBL
University of Chemistry and Technology Prague
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of rabbit microsomes SERCA1a by enzyme-coupled methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+4nMAssay Description:Inhibitory concentration against recombinant rat androgen receptor expressed in Escherichia coli using [3H]methyltrienolone (R 1881)More data for this Ligand-Target Pair
Affinity DataIC50: 1.46E+4nMAssay Description:Antagonist activity at full length human PXR transfected in human HepG2 cells assessed as reduction in rifaximin-induced receptor transactivation aft...More data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+4nMAssay Description:Inhibition of human GalE by HPAEC assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+4nMAssay Description:CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ...More data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+4nMAssay Description:CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)