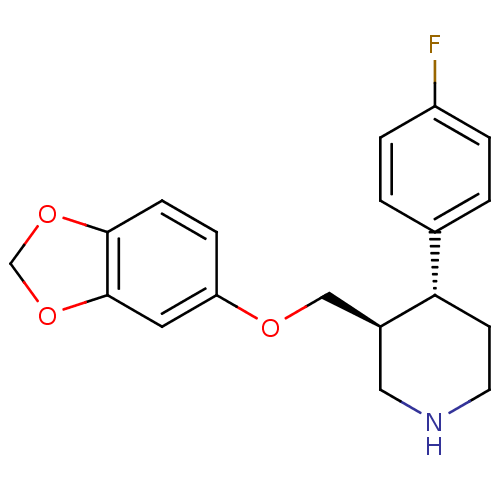
BDBM22416 (3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine::(3S,4R)-3-[(2H-1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine::CHEMBL490::PAROXETINE::US09969700, Paroxetine::US9944618, Compound ID No. 182::[3H]Paroxetine
SMILES c1cc(ccc1[C@@H]2CCNC[C@H]2COc3ccc4c(c3)OCO4)F
InChI Key InChIKey=AHOUBRCZNHFOSL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 145 hits for monomerid = 22416
Found 145 hits for monomerid = 22416
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0400nMAssay Description:Binding inhibition towards human serotonin transporterMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0770nMAssay Description:Evaluated for affinity at 5-HT uptake site using [3H]paroxetine as radioligand in radioligand binding assayMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0800nMAssay Description:Displacement of [125I]RTI55 binding from human wild type SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0900nMAssay Description:Displacement of [3H]paroxetine from SERT receptor in human platelet membraneMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.110nMAssay Description:Displacement of [3H]citalopram from human SERT expressed in HEK293 cell membranes after 1 hr by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 0.130nMAssay Description:Equilibrium dissociation constant (KD) for Competitive binding between [3H]- imipramine and the compound at human transporter -hSERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 0.150nMAssay Description:The potency of the [3H]paroxetine for 5-HT transportersMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Displacement of [125I]RTI-55 from human recombinant SERT expressed in HEK293 cells after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibition of [3H]paroxetine (0.2 nM) binding to 5-HT transporterMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.380nMAssay Description:Displacement of [3H]paroxetine from human SERT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.380nMAssay Description:Displacement of [3H]paroxetine from human SERT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.420nMAssay Description:Displacement of [3H]citalopram from human SERT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.420nMAssay Description:Displacement of [3H]citalopram from human SERT expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Tested in vitro for serotonin(5-HT) neuronal uptake inhibitionMore data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortexMore data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Compound was evaluated for its ability to displace [3H]citalopram binding to the rat cortical Serotonin transporterMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.560nMAssay Description:Inhibition of human SERT expressed in CHO cell membranes assessed as reduction in [3H]serotonin uptake preincubated for 10 mins followed by [3H]serot...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]-Citalopram from human SERT extracted from HEK293 cell membrane assessed as inhibition constant incubated for 60 mins by microbet...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Binding affinity to the serotonin transporter (SERT) measured by displacement of [3H]paroxetine in male wistar ratsMore data for this Ligand-Target Pair
Affinity DataKi: 0.730nMAssay Description:Inhibition of [3H]5-HT reuptake into rat frontal cortex synaptosomesMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:SERT: This protocol was designed to measure inhibition of uptake by the human serotonin transporter. The reagents were human SERT (HEK293F) cells, fl...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of serotonin uptake at human SERT expressed in JAR cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.43nMAssay Description:Inhibition of uptake of [3H]5-HT in synaptosomes from rat cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant MPO (unknown origin) assessed as reduction in taurine chloramine production preincubated with enzyme and taurine followed b...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Tested in vitro for norepinephrine (NE) neuronal uptake inhibitionMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Inhibition of [3H]- NE reuptake into rat hippocampal synaptosomesMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Human)
Hong Kong University of Science and Technology
Curated by ChEMBL
Hong Kong University of Science and Technology
Curated by ChEMBL
Affinity DataKd: 40nMAssay Description:Equilibrium dissociation constant (KD) for Competitive binding between [3H]- nisoxatine and the compound at human Norepinephrine transporterMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Human)
Hong Kong University of Science and Technology
Curated by ChEMBL
Hong Kong University of Science and Technology
Curated by ChEMBL
TargetSodium-dependent noradrenaline transporter(Human)
Hong Kong University of Science and Technology
Curated by ChEMBL
Hong Kong University of Science and Technology
Curated by ChEMBL
TargetSodium-dependent noradrenaline transporter(Human)
Hong Kong University of Science and Technology
Curated by ChEMBL
Hong Kong University of Science and Technology
Curated by ChEMBL
TargetSodium-dependent noradrenaline transporter(Human)
Hong Kong University of Science and Technology
Curated by ChEMBL
Hong Kong University of Science and Technology
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Binding inhibition towards human norepinephrine transporterMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)