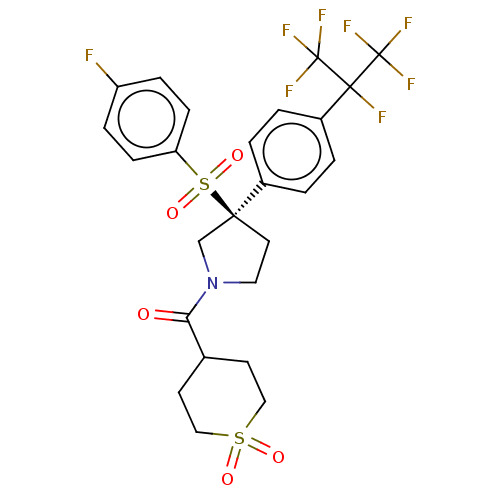
BDBM253525 US9458171, 485
SMILES Fc1ccc(cc1)S(=O)(=O)[C@]1(CCN(C1)C(=O)C1CCS(=O)(=O)CC1)c1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F
InChI Key InChIKey=ZOHVYFFYFCNUAZ-QFIPXVFZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 253525
Found 7 hits for monomerid = 253525
Affinity DataIC50: 54nMT: 2°CAssay Description:The binding of potential ligands to RORγ is measured by competition with [3H] 25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintill...More data for this Ligand-Target Pair
Affinity DataEC50: 33nMAssay Description:Inverse agonist activity at human Gal4-fused RORgammat LBD (267 to 516 residues) expressed in human Jurkat cells measured after 18 hrs by steady-glo ...More data for this Ligand-Target Pair
Affinity DataEC50: >4.00E+4nMAssay Description:Inverse agonist activity at human Gal4-fused RORalpha LBD (272 to 556 residues) expressed in human Jurkat cells measured after 18 hrs by steady-glo l...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataEC50: >5.00E+4nMAssay Description:Transactivation of PXR (unknown origin) expressed in human HepG2 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: >7.50E+3nMAssay Description:Transactivation of human GAL4-fused LXRalpha expressed in African green monkey CV1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: >7.50E+3nMAssay Description:Transactivation of human GAL4-fused LXRbeta expressed in African green monkey CV1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: >4.00E+4nMAssay Description:Inverse agonist activity at human Gal4-fused RORbeta LBD (200 to 559 residues) expressed in human Jurkat cells measured after 18 hrs by steady-glo lu...More data for this Ligand-Target Pair