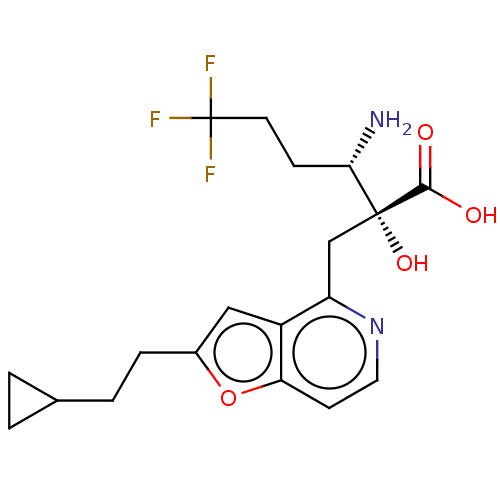
BDBM271318 US10059720, Example 133::US10975091, Example 133
SMILES N[C@@H](CCC(F)(F)F)[C@](O)(Cc1nccc2oc(CCC3CC3)cc12)C(O)=O
InChI Key InChIKey=ITNZADUFFYCMQI-FUHWJXTLSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 271318
Found 4 hits for monomerid = 271318
Affinity DataIC50: 1nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 7.10nMAssay Description:hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 7.10nMAssay Description:HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult...More data for this Ligand-Target Pair