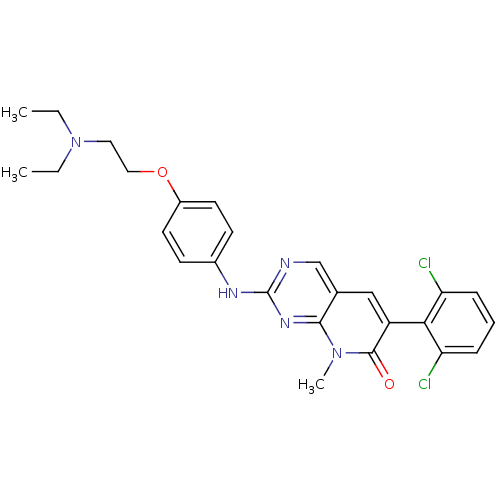
BDBM3096 2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv.::6-(2,6-Dichlorophenyl)-2-[4-(2-diethylaminoethoxy)phenylamino]-8-methyl-8H-pyrido[2,3-d]pyrimidin-7-one, Dihydrochloride::6-(2,6-dichlorophenyl)-2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-methyl-7H,8H-pyrido[2,3-d]pyrimidin-7-one::CHEMBL49120::D3RKN_84::PD166285
SMILES CCN(CC)CCOc1ccc(cc1)Nc2ncc3c(n2)N(C(=O)C(=C3)c4c(cccc4Cl)Cl)C
InChI Key InChIKey=IFPPYSWJNWHOLQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 43 hits for monomerid = 3096
Found 43 hits for monomerid = 3096
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Displacement of (6-FAM)KI(pY)VV from full length PKMYT1 (unknown origin) by fluorescence polarization immuno assayMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human full length PKMYT1 expressed in HEK293 cells using EFS (247 to 259 residues) as substrate after 1 hr by fluorescence polarization...More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of (6-FAM)KI(pY)VV from PKMYT1 kinase domain (unknown origin) by fluorescence polarization binding assayMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of N-[3',6'-dihydroxy-3-oxo-3H-spiro(2-benzofuran-1,9'-xanthen)-5-yl]-N'-[2-(4-{4-[N-(2-chloro-6-methylphenyl)-5-carboxamido]-thiazol-2-yl...More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of (6-FAM)KI(pY)VV from PKMYT1 kinase domain (unknown origin) by fluorescence polarization binding assayMore data for this Ligand-Target Pair
Affinity DataKd: 5nMAssay Description:Binding affinity to recombinant human N-terminal His6-tagged Wee2 kinase domain (202 to 492 residues) expressed in Escherichia coli BL21 (DE3) by iso...More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Competitive inhibition of human Myt1 by TR-FRET assayMore data for this Ligand-Target Pair
TargetMyelin transcription factor 1(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 7.20nMAssay Description:Binding affinity to human full-length His-tagged Myt1 kinase expressed in HEK293 cells by TR-FRET based binding assayMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Displacement of (6-FAM)KI(pY)VV from full length PKMYT1 (unknown origin) by fluorescence polarization immuno assayMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human full length PKMYT1 expressed in HEK293 cells using EFS (247 to 259 residues) as substrate after 1 hr by fluorescence polarization...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of chicken c-Src tyrosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataKd: 11nMAssay Description:Binding affinity to recombinant human N-terminal His6-tagged Wee1 kinase domain (291 to 575 residues) expressed in Escherichia coli BL21 (DE3) by iso...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 2(Human)
University of Houston
Curated by ChEMBL
University of Houston
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant RIPK2 (unknown origin) using RS repeat peptide as substrate preincubated for 5 mins followed by substrate and ATP addition ...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of human ALK2 using casein as substrate incubated for 30 mins in presence of [gamma33P]ATP by radiometric hotspot kinase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of full length WEE1 (unknown origin) assessed as reduction in cdc2/cyclin B phosphorylation using biotinylated histone H1 peptide (PKTPKKA...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of Wee1 (unknown origin)More data for this Ligand-Target Pair
TargetMyelin transcription factor 1(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of MYT1 (unknown origin)More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibition of N-[3',6'-dihydroxy-3-oxo-3H-spiro(2-benzofuran-1,9'-xanthen)-5-yl]-N'-[2-(4-{4-[N-(2-chloro-6-methylphenyl)-5-carboxamido]-thiazol-2-yl...More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Displacement of (6-FAM)KI(pY)VV from PKMYT1 kinase domain (unknown origin) by fluorescence polarization binding assayMore data for this Ligand-Target Pair
TargetNucleotide-binding oligomerization domain-containing protein 2(Human)
University of Houston
Curated by ChEMBL
University of Houston
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of human NOD2 expressed in HEK-blue cells coexpressing NFkappaB-SEAP reporter assessed as reduction in L18-MDP-induced NF-kappaB activatio...More data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inhibition of Wee1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inhibition of CHK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 96nMAssay Description:Inhibitory concentration of compound against mouse Platelet-derived growth factor receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of CDK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of MYT1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of c-SRC (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of FGFR1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of PDGFR-beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 165nMAssay Description:Inhibition of checkpoint kinase Wee1More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKd: 247nMAssay Description:Binding affinity to recombinant human N-terminal His6-tagged Myt1 kinase domain (75 to 361 residues) by isothermal titration calorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of human epidermal growth factor receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+4nMAssay Description:Inhibition of Epidermal growth factor receptorMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)