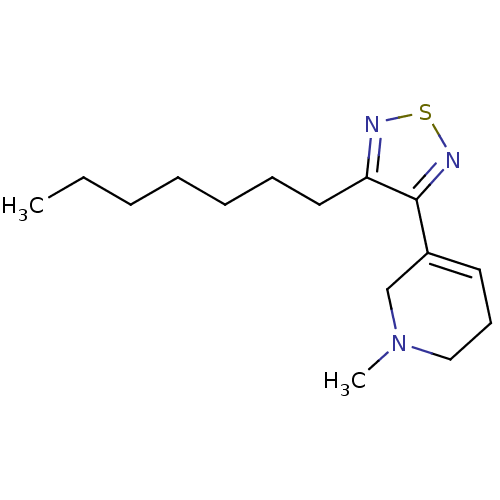
BDBM50003350 5-(4-Heptyl-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2,3,6-tetrahydro-pyridine::5-(4-Heptyl-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2,3,6-tetrahydro-pyridine; oxalic acid::CHEMBL120399::CHEMBL307837
SMILES CCCCCCCc1nsnc1C1=CCCN(C)C1
InChI Key InChIKey=KFMGSFKZOWXTCJ-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50003350
Found 5 hits for monomerid = 50003350
Affinity DataIC50: 73nMAssay Description:In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 187nMAssay Description:In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 187nMAssay Description:In vitro binding affinity against rat hippocampus M1 receptor using [3H]-pirenzepine (Pz) as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 224nMAssay Description:Efficacy at muscarinic acetylcholine receptor M1 measured by the ability to inhibit the electrically stimulated twitch of the rabbit vas deferensMore data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:Displacement of [3H]-pirenzepine (Pz) from rat hippocampus muscarinic acetylcholine receptor M1More data for this Ligand-Target Pair