BDBM50004529 CHEMBL2216859
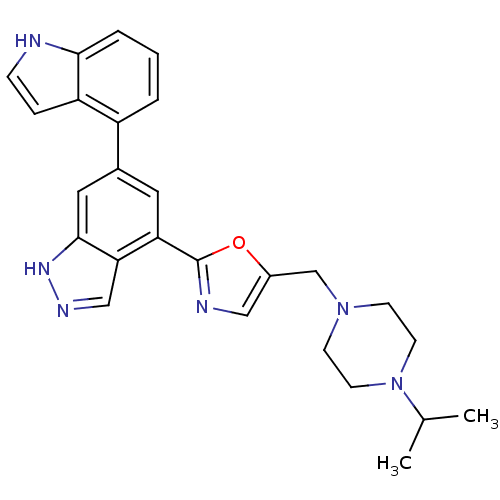
SMILES CC(C)N1CCN(CC1)Cc2cnc(o2)c3cc(cc4c3cn[nH]4)c5cccc6c5cc[nH]6
InChI Key InChIKey=MCIDWGZGWVSZMK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50004529
Found 5 hits for monomerid = 50004529
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Human)
Glaxosmithkline R&D
Curated by ChEMBL
Glaxosmithkline R&D
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins by HT...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Human)
Glaxosmithkline R&D
Curated by ChEMBL
Glaxosmithkline R&D
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of PI3KdeltaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Human)
Glaxosmithkline R&D
Curated by ChEMBL
Glaxosmithkline R&D
Curated by ChEMBL
Affinity DataIC50: 1.59E+3nMAssay Description:Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins by HTR...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Human)
Glaxosmithkline R&D
Curated by ChEMBL
Glaxosmithkline R&D
Curated by ChEMBL
Affinity DataIC50: 5.01E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins by HT...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Human)
Glaxosmithkline R&D
Curated by ChEMBL
Glaxosmithkline R&D
Curated by ChEMBL
Affinity DataIC50: 6.31E+3nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins by HT...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)