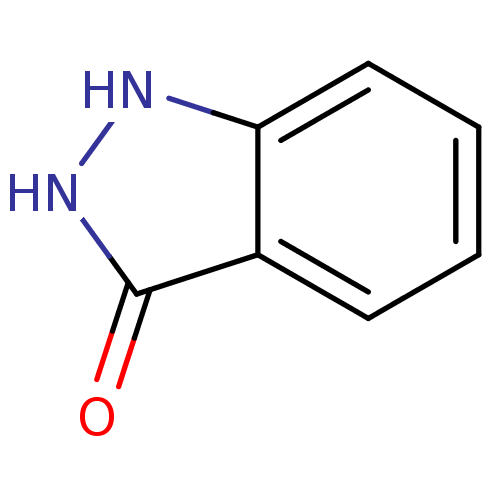
BDBM50008990 1,2-Dihydro-indazol-3-one::1H-indazol-3-ol::CHEMBL276725
SMILES O=c1[nH][nH]c2ccccc12
InChI Key InChIKey=SWEICGMKXPNXNU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50008990
Found 7 hits for monomerid = 50008990
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of recombinant NOS1 assessed as citrulline formationMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of nNOS in LPS-stimulated Wistar rat striata assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by liquid scintillati...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Rattus norvegicus)
Ici Pharmaceuticals Group
Curated by ChEMBL
Ici Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:In vitro inhibition of LTB4 production was measured in rat bloodMore data for this Ligand-Target Pair
Affinity DataKd: 4.43E+3nMAssay Description:Binding affinity to pig kidney DAAO by ITC methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:In vitro inhibition of PGE-2 production was measured in rat bloodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Ici Pharmaceuticals Group
Curated by ChEMBL
Ici Pharmaceuticals Group
Curated by ChEMBL
Affinity DataEC50: >7.45E+5nMAssay Description:Ex vivo inhibition of LTB4 production was measured in dog bloodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Ici Pharmaceuticals Group
Curated by ChEMBL
Ici Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 700nMAssay Description:In vitro inhibition of LTB4 (LT) production in dog bloodMore data for this Ligand-Target Pair