BDBM50010462 CHEBI:78825::CHEMBL3264002::US11147816, RO5126766-(CH5126766)::US11701360, R05126766::US11964950, Compound Ref-5::US20230270730, Compound ref-5::US20230382863, Compound avutometinib::US20240327355, Compound ref-5
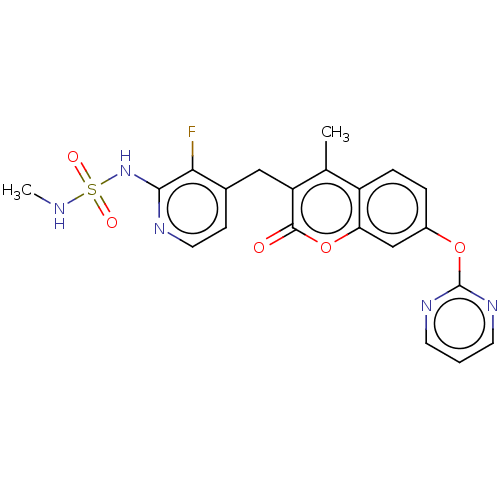
SMILES CC1=C(C(=O)Oc2c1ccc(c2)Oc3ncccn3)Cc4ccnc(c4F)NS(=O)(=O)NC
InChI Key InChIKey=LMMJFBMMJUMSJS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 50010462
Found 19 hits for monomerid = 50010462
TargetMitogen-activated protein kinase kinase kinase 1/RAF proto-oncogene serine/threonine-protein kinase(Human)
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataKd: 2.22nMAssay Description:Table 11: The experiments were performed on Biacore S200 (Cytiva). Biotinylated, unphosphorylated MEK1 was diluted to 0.1 uM by SPR buffer C (20 mM T...More data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:Inhibition of human BRAF V600E mutant using 5-Fl-SGQLIDSMANSFV-NH2 peptide as substrate by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The BRAF-inhibiting activity of the compounds listed in Table 6 below was evaluated by the time-resolved fluorescence-fluorescence resonance energy t...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The BRAF-inhibiting activity of the compounds listed in Table 3 below was evaluated by the time-resolved fluorescence-fluorescence resonance energy t...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The test compound, BRAF (Eurofins Genomics KK.) and MEK1 (Thermo Fisher Scientific Inc.) were mixed in ATP-containing buffer and reacted for 90 minut...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of wild type human BRAF using 5-Fl-SGQLIDSMANSFV-NH2 peptide as substrate by FRET assayMore data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Human)
Zhengzhou University
Curated by ChEMBL
Zhengzhou University
Curated by ChEMBL
Affinity DataIC50: 56nMAssay Description:Inhibition of human CRAF using 5-Fl-SGQLIDSMANSFV-NH2 peptide as substrate by FRET assayMore data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Human)
Zhengzhou University
Curated by ChEMBL
Zhengzhou University
Curated by ChEMBL
Affinity DataIC50: 56nMAssay Description:Inhibition of truncated active C-Raf (unknown origin) assessed as MEK1 phosphorylation using inactive MEK1 K97R as substrate after 45 minsMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Human)
Ghent University
Curated by ChEMBL
Ghent University
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibition of MEK1 (unknown origin)More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Human)
Ghent University
Curated by ChEMBL
Ghent University
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:The IC50 for MEK1 and 2 can be measured by methods in references such as [Yamaguchi et al. (2011) International Journal of Oncology 39:23-31].More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Human)
Ghent University
Curated by ChEMBL
Ghent University
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibition of MEK1 (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 292nMAssay Description:The MEK1-inhibiting activity of the compounds listed in Table 3 below were evaluated by the fluorescent polarization method as described below.The te...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 292nMAssay Description:The MEK1-inhibiting activity of the compounds listed in Table 6 below were evaluated by the fluorescent polarization method as described below. The t...More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Human)
Ghent University
Curated by ChEMBL
Ghent University
Curated by ChEMBL
Affinity DataIC50: 292nMAssay Description:The test compound, CRAF (Thermo Fisher Scientific Inc.), MEK1 (Thermo Fisher Scientific Inc.) and ERK2 (Carna Biosciences, Inc.) were mixed in ATP-co...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate measured after 30 mins preincubation by LC-MS/MS analysis in absence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate measured after 30 mins preincubation by LC-MS/MS analysis in presence of...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate measured after 30 mins preincubation by LC-MS/MS analysis in absence of N...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate measured after 30 mins preincubation by LC-MS/MS analysis in presence of ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)