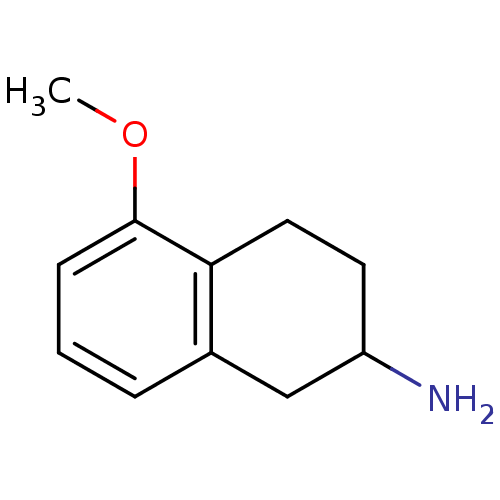
BDBM50020498 5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-ylamine::CHEMBL30560
SMILES COc1cccc2CC(N)CCc12
InChI Key InChIKey=SIHPGAYIYYGOIP-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50020498
Found 4 hits for monomerid = 50020498
Affinity DataKi: 334nMAssay Description:Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D2L of CHO K-1 cells.More data for this Ligand-Target Pair
Affinity DataKi: 683nMAssay Description:Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D3 expressed on CHO K-1 cells.More data for this Ligand-Target Pair
Affinity DataKi: >3.33E+4nMAssay Description:Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D4.2 of CHO K-1 cells.More data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Bos taurus (bovine))
University Of Kansas
Curated by ChEMBL
University Of Kansas
Curated by ChEMBL
Affinity DataKi: 1.57E+5nMAssay Description:In vitro inhibitory activity against bovine phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair