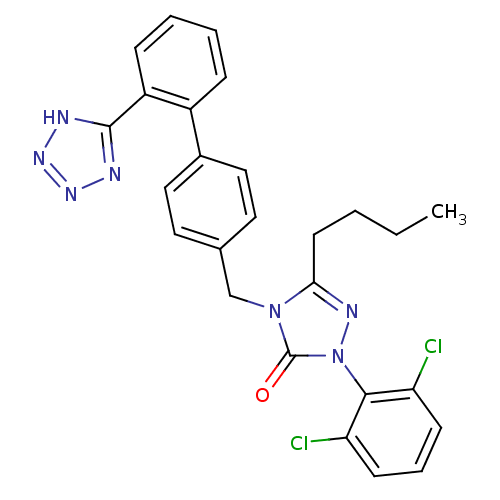
BDBM50044479 5-Butyl-2-(2,6-dichloro-phenyl)-4-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-2,4-dihydro-[1,2,4]triazol-3-one::CHEMBL278741
SMILES CCCCc1nn(-c2c(Cl)cccc2Cl)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1
InChI Key InChIKey=UUYKNTFLYAPMIE-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50044479
Found 4 hits for monomerid = 50044479
Affinity DataIC50: 5.80nMAssay Description:Displacement of 125I[Sar,Ile] from rabbit aorta membrane Angiotensin II receptor type 1More data for this Ligand-Target Pair
Affinity DataIC50: 5.80nMAssay Description:Antagonist activity at angiotensin 2 type-1 receptor in Oryctolagus cuniculus (rabbit) aorta in presence of bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80nMAssay Description:Antagonist activity at angiotensin 2 type-1 receptor in Oryctolagus cuniculus (rabbit) aorta in presence of bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80nMAssay Description:In vitro antagonistic activity for angiotensin II receptor, type 1 by displacing 125I[Sar, ILe8 ]AII radioligand in rabbit aorta membrane without usi...More data for this Ligand-Target Pair