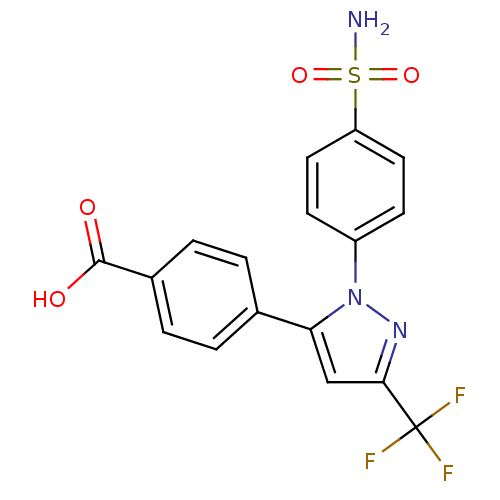
BDBM50057540 4-[2-(4-Sulfamoyl-phenyl)-5-trifluoromethyl-2H-pyrazol-3-yl]-benzoic acid::CHEMBL743::Carboxylic acid metabolite of celecoxib
SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(cc1)C(O)=O)C(F)(F)F
InChI Key InChIKey=WTHNOVFEXONZMI-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50057540
Found 4 hits for monomerid = 50057540
Affinity DataIC50: 1.12E+4nMAssay Description:In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2)More data for this Ligand-Target Pair
Affinity DataIC50: 1.12E+4nMAssay Description:Inhibitory activity against prostaglandin G/H synthase 2 (COX-2)More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory concentration required to block recombinant human prostaglandin G/H synthase 1 (COX-1)More data for this Ligand-Target Pair
Affinity DataIC50: 2.51E+5nMAssay Description:Inhibitory activity against prostaglandin G/H synthase 1 (COX-1)More data for this Ligand-Target Pair