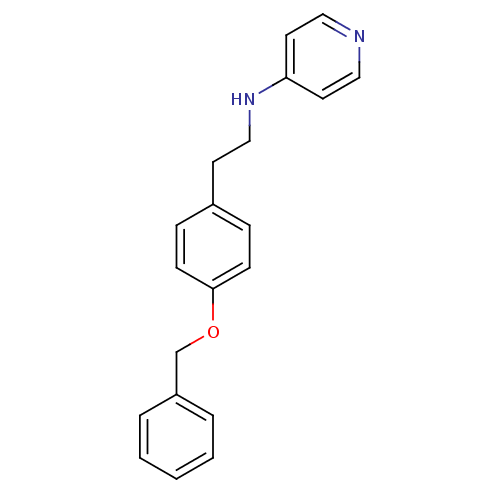
BDBM50070788 CHEMBL48029::N-(4-(benzyloxy)phenethyl)pyridin-4-amine::[2-(4-Benzyloxy-phenyl)-ethyl]-pyridin-4-yl-amine
SMILES C(Cc1ccc(OCc2ccccc2)cc1)Nc1ccncc1
InChI Key InChIKey=WPBRSJXCRWSAHJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50070788
Found 6 hits for monomerid = 50070788
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 93nMAssay Description:Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cellsMore data for this Ligand-Target Pair
Affinity DataKi: 7.90E+3nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.68E+5nMAssay Description:Compound was evaluated for its inhibitory activity against trypsinMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 102nMAssay Description:Antagonist activity at human NR2B expressed in Ltk- cells by calcium flux assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Displacement of [35S]MK499 from hERG potassium channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Activity at human NR2A expressed in Ltk- cells by calcium flux assayMore data for this Ligand-Target Pair