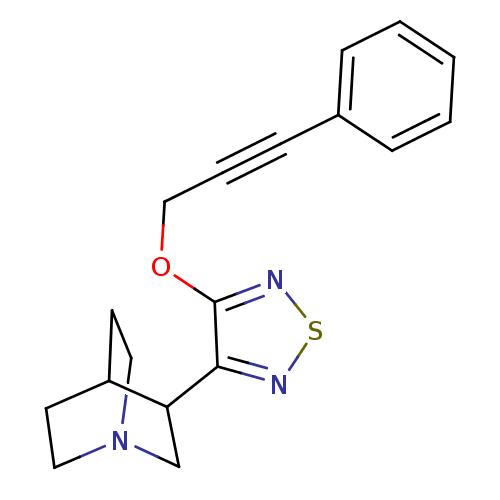
BDBM50072228 3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3-yl]-1-aza-bicyclo[2.2.2]octane::CHEMBL99521::NNC 11-1314
SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1
InChI Key InChIKey=SNSMIIQEKVYNOH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50072228
Found 8 hits for monomerid = 50072228
Affinity DataIC50: 4.70nMAssay Description:Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligandMore data for this Ligand-Target Pair
Affinity DataEC50: 16nMAssay Description:Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 62nMAssay Description:Stimulation of cAMP in CHO cells expressing human m2 receptorMore data for this Ligand-Target Pair