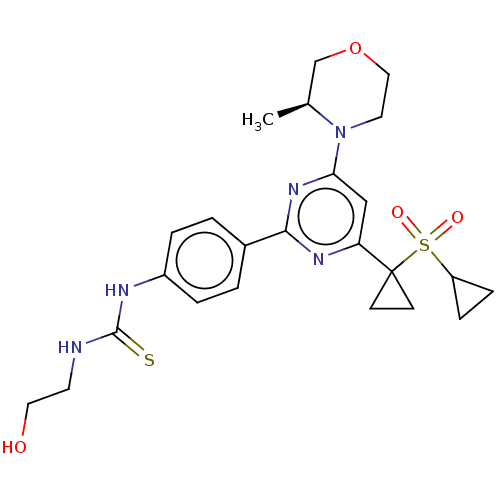
BDBM50072961 CHEMBL3410672
SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1ccc(NC(=S)NCCO)cc1)C1(CC1)S(=O)(=O)C1CC1
InChI Key InChIKey=JWGVUDPAMQEIJU-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50072961
Found 5 hits for monomerid = 50072961
Affinity DataIC50: 1.5nMAssay Description:Inhibition of recombinant truncated FLAG-tagged mTOR (1362 to 2549 aa) (unknown origin) expressed in HEK293 cells using biotinylated p70 peptide as s...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Human)
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 912nMAssay Description:Inhibition of recombinant PI3Kalpha (unknown origin) using biotinylated PIP2 as substrateMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Human)
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of recombinant PI3Kbeta (unknown origin) using biotinylated PIP2 as substrateMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Human)
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 6.31E+3nMAssay Description:Inhibition of recombinant PI3Kgamma (unknown origin) using biotinylated PIP2 as substrateMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Human)
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 9.33E+3nMAssay Description:Inhibition of recombinant PI3Kdelta (unknown origin) using biotinylated PIP2 as substrateMore data for this Ligand-Target Pair