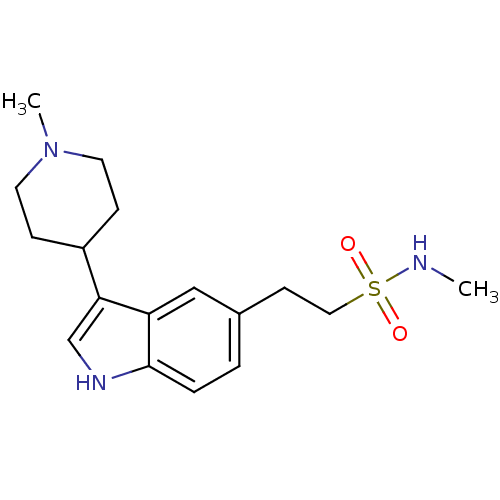
BDBM50073682 CHEMBL1278::N-methyl-2-(3-(1-methylpiperiden-4-yl)indole-5-yl)ethanesulfonamide::N-methyl-2-[3-(1-methyl-4-piperidyl)-1H-indol-5-yl]-ethanesulfonamide::N-methyl-2-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]ethanesulfonamide::NARATRIPTAN
SMILES CNS(=O)(=O)CCc1ccc2[nH]cc(C3CCN(C)CC3)c2c1
InChI Key InChIKey=AMKVXSZCKVJAGH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50073682
Found 9 hits for monomerid = 50073682
Affinity DataKi: 1.5nMAssay Description:Binding affinity to 5-HT1B receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Binding affinity to 5-HT1DR (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataEC50: 1.60nMAssay Description:Ability to inhibit forskolin-stimulated adenylate cyclase in a cell line expressing human 5-hydroxytryptamine 1D receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1B receptorMore data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1A receptorMore data for this Ligand-Target Pair