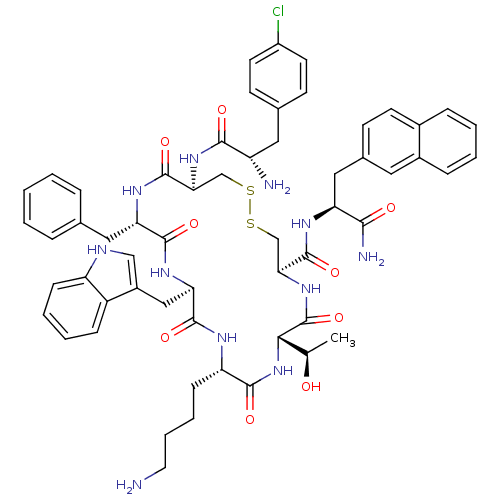
BDBM50077308 10-(4-Amino-butyl)-19-[2-amino-3-(4-chloro-phenyl)-propionylamino]-16-benzyl-7-(1-hydroxy-ethyl)-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaaza-cycloicosane-4-carboxylic acid (1-carbamoyl-2-naphthalen-2-yl-ethyl)-amide::CHEMBL439005
SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(N)=O)NC(=O)[C@@H](N)Cc1ccc(Cl)cc1
InChI Key InChIKey=VSRBFZFTFFYHBT-SLNGEIHESA-N
Data 5 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50077308
Found 5 hits for monomerid = 50077308
TargetSomatostatin receptor type 2(Homo sapiens (Human))
Tulane University School Of Medicine
Curated by ChEMBL
Tulane University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 51nMAssay Description:The compound was tested for binding affinity against human Somatostatin receptor type 2More data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Tulane University School Of Medicine
Curated by ChEMBL
Tulane University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 87nMAssay Description:The compound was tested for binding affinity against human Somatostatin receptor type 5More data for this Ligand-Target Pair
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Tulane University School Of Medicine
Curated by ChEMBL
Tulane University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 290nMAssay Description:The compound was tested for binding affinity against human Somatostatin receptor type 3More data for this Ligand-Target Pair
TargetSomatostatin receptor type 1(Homo sapiens (Human))
Tulane University School Of Medicine
Curated by ChEMBL
Tulane University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 443nMAssay Description:The compound was tested for binding affinity against human Somatostatin receptor type 1More data for this Ligand-Target Pair
TargetSomatostatin receptor type 4(Homo sapiens (Human))
Tulane University School Of Medicine
Curated by ChEMBL
Tulane University School Of Medicine
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:The compound was tested for binding affinity against human Somatostatin receptor type 4More data for this Ligand-Target Pair