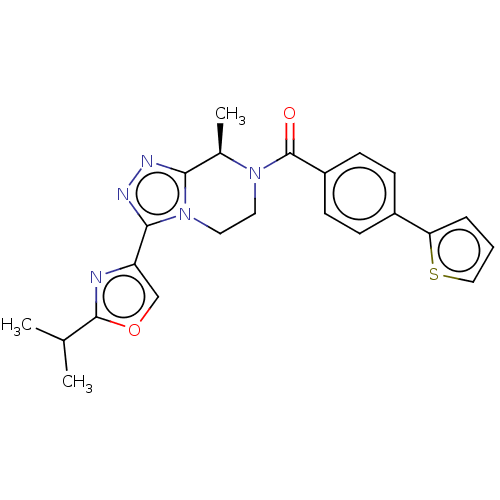
BDBM50081196 CHEMBL3422015::US10065961, Compound 13::US10683295, Compound 13::US10941151, Compound 13::US9475814, 13
SMILES CC(C)c1nc(co1)-c1nnc2[C@@H](C)N(CCn12)C(=O)c1ccc(cc1)-c1cccs1
InChI Key InChIKey=GYGRAOHPQAELJF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 50081196
Found 19 hits for monomerid = 50081196
Affinity DataIC50: 2nMAssay Description:The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Changes in intracellular calcium levels are a recognized indicator of G protein-coupled receptor activity. The efficacy of compounds of the invention...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad...More data for this Ligand-Target Pair
Affinity DataKi: 3nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit...More data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Antagonist activity at recombinant human NK3R expressed in CHO cells assessed as inhibition of NKB-induced Ca2+ signaling by aequorin Ca2+ biolumines...More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of [3H]-SB222200 from monkey NK3R after 90 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Displacement of [3H]-SB222200 from rat NK3R after 90 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Displacement of [3H]-SB222200 from rat NK3R after 90 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells after 5 mins by patch-clamp assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.94E+3nMAssay Description:Displacement of [3H]-Substance P from human NK1R after 90 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Displacement of [125I]-neurokinin A from human NK2R after 90 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin) by luciferase reporter assay in presence of NADPH regeneration systemMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin) by luciferase reporter assay in presence of NADPH regeneration systemMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) by luciferase reporter assay in presence of NADPH regeneration systemMore data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin) by luciferase reporter assay in presence of NADPH regeneration systemMore data for this Ligand-Target Pair
Affinity DataIC50: 8.10E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin) by luciferase reporter assay in presence of NADPH regeneration systemMore data for this Ligand-Target Pair