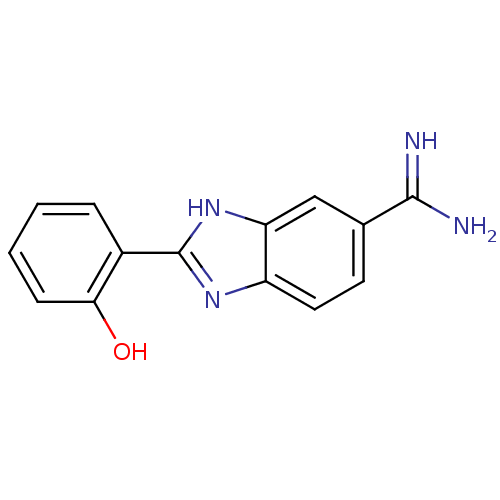
BDBM50100897 2-(2-Hydroxy-phenyl)-1H-benzoimidazole-5-carboxamidine::2-(2-Hydroxy-phenyl)-3H-benzoimidazole-5-carboxamidine::2-{5-[AMINO(IMINIO)METHYL]-1H-BENZIMIDAZOL-2-YL}BENZENOLATE::CHEMBL433501
SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1ccccc1O
InChI Key InChIKey=URJKRCBBKTXOHS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50100897
Found 14 hits for monomerid = 50100897
Affinity DataKi: 5.50E+3nMAssay Description:Inhibition of urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+3nMAssay Description:ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA).More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+3nMAssay Description:Inhibition of human uPA by fluorescence based assay using L-PyroGlu-Gly-Arg-pNA.HCl as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Binding affinity against human coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Inhibition of bovine factor 10a by fluorescence based assay using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Inhibition of Human Serine Protease Trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Inhibition of human trypsin by fluorescence based assay using 25 uM Boc-Gln-Ala-Arg-7-amido-4-methyl coumarinhydrobromide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+4nMAssay Description:Inhibition of human recombinant hepsin by fluorescence based assay using 65 uM BOC-Gln-Arg-Arg -AMC as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 4.60E+4nMAssay Description:Inhibition of Human Serine Protease Plasmin.More data for this Ligand-Target Pair
Affinity DataKi: 4.60E+4nMAssay Description:Inhibition of human plasmin by fluorescence based assay using pyroGlu-Phe-Lys-pNA.HCl as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nMAssay Description:Inhibition of human thrombin by fluorescence based assay using 100 uM Boc-Gln-Ala-Arg-7-amido-4-methyl coumarinhydrobromide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nMAssay Description:Inhibition of Human Serine Protease Thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 7.50E+4nMAssay Description:Inhibition of Human Serine Protease tissue type Plasminogen Activator (t-PA).More data for this Ligand-Target Pair
TargetSporulation kinase A(Bacillus subtilis (strain 168))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.70E+5nMAssay Description:Inhibition of KinA/Sp0F system two component (TCS) from Bacillus subtilis.More data for this Ligand-Target Pair