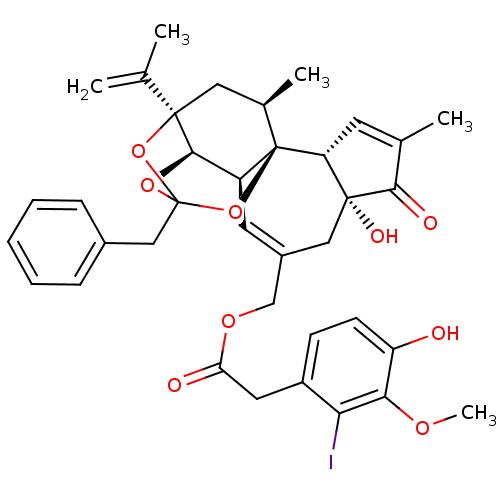
BDBM50111692 13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5-oxo-(1R,6R,13S,15R,17R)-12,14,18-trioxapentacyclo[11.4.1.01,10.02,6.011,15]octadeca-3,8-dien-8-ylmethyl 2-(4-hydroxy-2-iodo-3-methoxyphenyl)acetate::CHEMBL18246
SMILES COc1c(O)ccc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)c1I
InChI Key InChIKey=IMTPTSBICCCMPL-ZQSPPQLISA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50111692
Found 2 hits for monomerid = 50111692
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of [3H]RTX binding to human Vanilloid receptor subtype 1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Induced [Ca2+] influx in Vanilloid receptor subtype 1 expressing HEK 293 cellsMore data for this Ligand-Target Pair