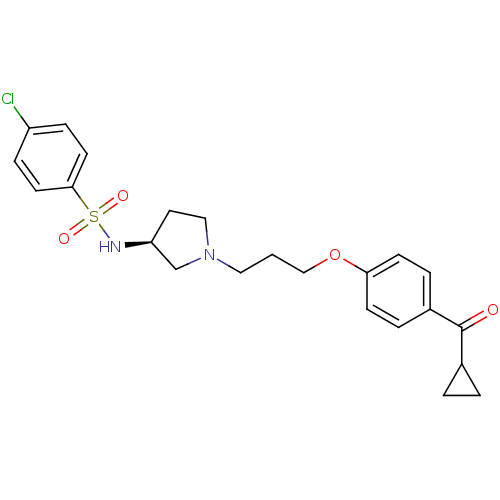
BDBM50119728 4-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenoxy)-propyl]-pyrrolidin-3-yl}-benzenesulfonamide::CHEMBL104344
SMILES Clc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1
InChI Key InChIKey=BTCOIJOGNYPDOY-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50119728
Found 3 hits for monomerid = 50119728
Affinity DataKi: 3.80nMAssay Description:Binding affinity towards rats Histamine type 3 (H3) receptorMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Binding affinity towards human Histamine H2 receptor (For compound 11)More data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Binding affinity to the human Histamine H1 receptorMore data for this Ligand-Target Pair