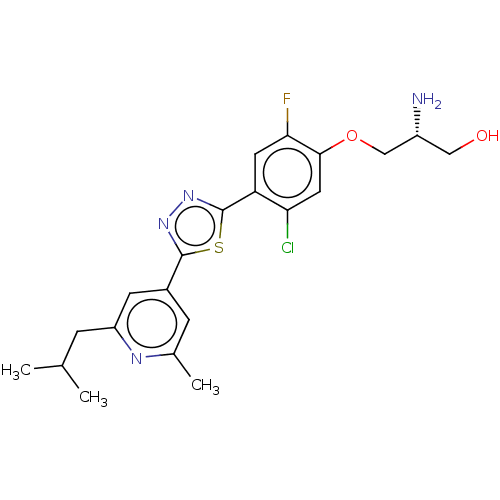
BDBM50136346 CHEMBL3754270
SMILES CC(C)Cc1cc(cc(C)n1)-c1nnc(s1)-c1cc(F)c(OC[C@H](N)CO)cc1Cl
InChI Key InChIKey=ZMDMBZLDKVZLCF-CQSZACIVSA-N
Data 1 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50136346
Found 1 hit for monomerid = 50136346
TargetSphingosine 1-phosphate receptor 1(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 0.800nMAssay Description:Agonist activity at human S1P1 receptor expressed in EDG1-bla U2OS cells incubated for 18 hrs prior to GenBlazer substrate addition by beta-arrestin ...More data for this Ligand-Target Pair