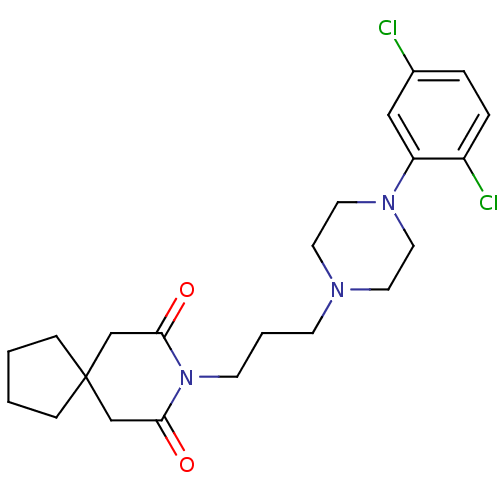
BDBM50143682 8-{3-[4-(2,5-Dichloro-phenyl)-piperazin-1-yl]-propyl}-8-aza-spiro[4.5]decane-7,9-dione::CHEMBL55319
SMILES Clc1ccc(Cl)c(c1)N1CCN(CCCN2C(=O)CC3(CCCC3)CC2=O)CC1
InChI Key InChIKey=SYPZJTHVWQUCJS-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50143682
Found 4 hits for monomerid = 50143682
Affinity DataKi: 1.72nMAssay Description:Binding affinity to the adrenergic receptor alpha-1D of rat aortaMore data for this Ligand-Target Pair
Affinity DataKi: 2.85nMAssay Description:In vitro binding affinity towards alpha-1A adrenergic receptor through displacement of [3H]-prazosin in epididymal rat vas deferens.More data for this Ligand-Target Pair
Affinity DataKi: 13.6nMAssay Description:Binding affinity constant against alpha-1B adrenergic receptor of guinea pig spleenMore data for this Ligand-Target Pair
Affinity DataKi: 401nMAssay Description:Binding affinity to human 5-hydroxytryptamine 1A receptor was measured using [3H]-8-hydroxy-2-(di-n-propylamine) tetraline [3H]-8-OH-DPAT) as radioli...More data for this Ligand-Target Pair