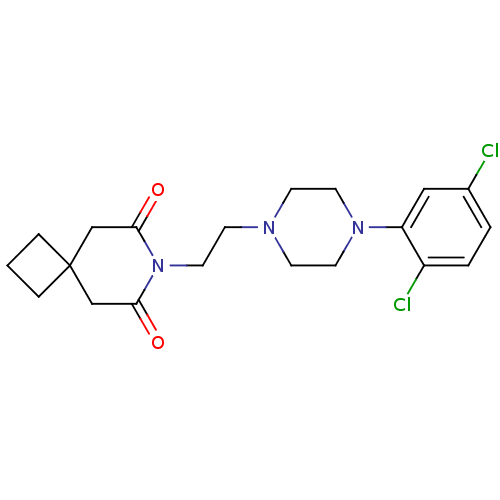
BDBM50143716 7-{2-[4-(2,5-Dichloro-phenyl)-piperazin-1-yl]-ethyl}-7-aza-spiro[3.5]nonane-6,8-dione::CHEMBL55882
SMILES Clc1ccc(Cl)c(c1)N1CCN(CCN2C(=O)CC3(CCC3)CC2=O)CC1
InChI Key InChIKey=VRQYVXIQZGEPQK-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50143716
Found 4 hits for monomerid = 50143716
Affinity DataKi: 2.65nMAssay Description:Binding affinity to the adrenergic receptor alpha-1D of rat aortaMore data for this Ligand-Target Pair
Affinity DataKi: 79.6nMAssay Description:Binding affinity constant against alpha-1B adrenergic receptor of guinea pig spleenMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:In vitro binding affinity towards alpha-1A adrenergic receptor through displacement of [3H]-prazosin in epididymal rat vas deferens.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Binding affinity to human 5-hydroxytryptamine 1A receptor was measured using [3H]-8-hydroxy-2-(di-n-propylamine) tetraline [3H]-8-OH-DPAT) as radioli...More data for this Ligand-Target Pair