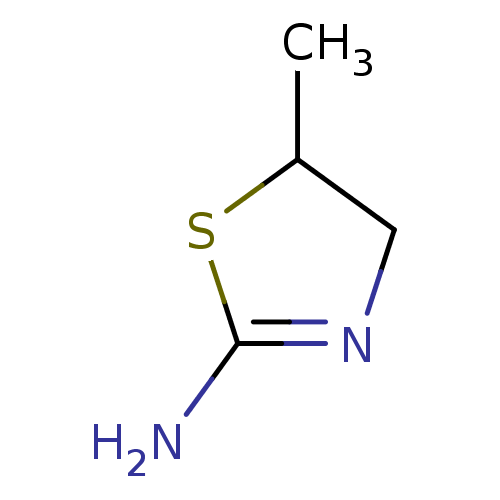
BDBM50150913 5-Methyl-thiazolidin-(2E)-ylideneamine::CHEMBL185505
SMILES CC1CN=C(N)S1
InChI Key InChIKey=IKFLLHVVKJTHAH-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50150913
Found 3 hits for monomerid = 50150913
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibitory activity against Inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibitory activity against Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibitory activity against Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair