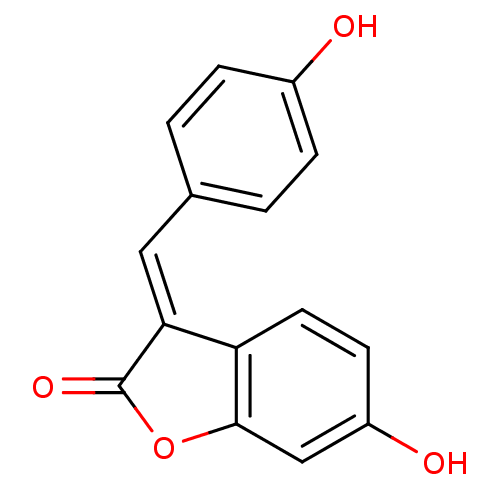
BDBM50164888 6-Hydroxy-3-[1-(4-hydroxy-phenyl)-meth-(E)-ylidene]-3H-benzofuran-2-one::CHEMBL195416::isoaurostatin
SMILES Oc1ccc(\C=C2\C(=O)Oc3cc(O)ccc23)cc1
InChI Key InChIKey=DJGNNZVFOBIPMK-NTUHNPAUSA-N
Data 21 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 50164888
Found 21 hits for monomerid = 50164888
Affinity DataIC50: 3.07E+5nMAssay Description:Inhibition calf thymus gland topoisomerase 1 assessed as conversion of supercoiled pBR322 DNA to relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 3.07E+5nMAssay Description:Inhibition of calf thymus gland topoisomerase 1 mediated DNA relaxationMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition topoisomerase 1 in african green monkey Vero cells assessed as conversion of supercoiled pBR322 DNA to relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition topoisomerase 1 in human A549 cells assessed as conversion of supercoiled pBR322 DNA to relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition topoisomerase 1 in human COLO201 cells assessed as conversion of supercoiled pBR322 DNA to relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition topoisomerase 1 in human HeLa cells assessed as conversion of supercoiled pBR322 DNA to relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition human placenta topoisomerase 2 assessed as conversion of supercoiled pBR322 DNA to relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition human placenta topoisomerase 2 assessed as conversion of catenated kinetoplast DNA to minicircle monomerMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of bovine pancreas RNase A assessed as undigested supercoiled pBR322 DNA concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of bovine pancreas DNase 1 assessed as undigested supercoiled pBR322 DNA concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of porcine spleen DNase 2 assessed as undigested supercoiled pBR322 DNA concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of T4 ligase from bacteriophage infected Escherichia coli assessed as ligation of supercoiled pBR322 DNAMore data for this Ligand-Target Pair
TargetType II restriction enzyme BamHI(Bacillus amyloliquefaciens)
Kumamoto University
Curated by ChEMBL
Kumamoto University
Curated by ChEMBL
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of Bacillus amyloliquifaction Bam H1 assessed as undigested supercoiled pBR322 DNA concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of Escherichia coli Eco R1 assessed as undigested supercoiled pBR322 DNA concentrationMore data for this Ligand-Target Pair
TargetType II restriction enzyme HindIII(Haemophilus influenzae)
Kumamoto University
Curated by ChEMBL
Kumamoto University
Curated by ChEMBL
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of Haemophilus influenzae Hind 3 assessed as undigested supercoiled pBR322 DNA concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of Providencia stuartii Pst 1 assessed as undigested supercoiled pBR322 DNA concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of Streptomyces caespitosus Sca 1 assessed as undigested supercoiled pBR322 DNA concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition of telomerase from human COLO201 cellMore data for this Ligand-Target Pair
Affinity DataIC50: 3.07E+5nMAssay Description:Inhibitory concentration against DNA topoisomerase I activityMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibitory concentration against DNA topoisomerase II activityMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibition topoisomerase 1 in mouse NIH/3T3 cells assessed as conversion of supercoiled pBR322 DNA to relaxed formMore data for this Ligand-Target Pair