BDBM50166289 CHEBI:43645::CHEMBL216543
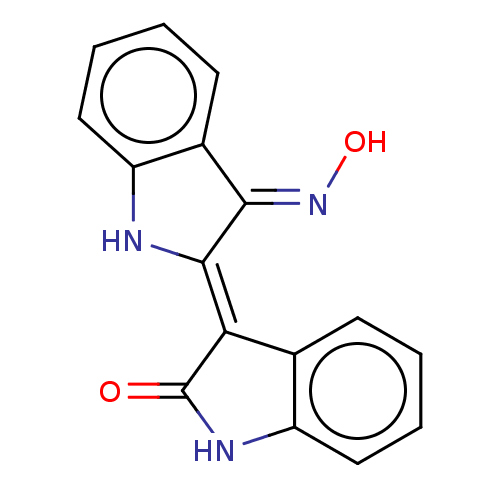
SMILES c1ccc2c(c1)/C(=C/3\C(=N\O)\c4ccccc4N3)/C(=O)N2
InChI Key InChIKey=HBDSHCUSXQATPO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 50166289
Found 21 hits for monomerid = 50166289
Affinity DataIC50: 22nMAssay Description:Inhibition of GSK-3beta (unknown origin) expressed in Sf21 cellsMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Rat)
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of recombinant recombinant rat GSK3beta using GS-1 peptide as substrate in presence of [gamma-32P]ATP measured after 30 mins incubation by...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of GSK3beta (unknown origin) assessed as decrease in tau phosphorylation at Ser214 residue incubated for 30 mins by Western blot assayMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta/[Tau protein] kinase(Pig)
Zunyi Medical University
Curated by ChEMBL
Zunyi Medical University
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of porcine brain GSK-3alpha/beta incubated for 30 mins in presence of [33p]-gamma ATP by scintillation counter analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of recombinant FLT3 (unknown origin) in presence of ATP by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of RSK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of GST-fused CDK5/p25 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of CDK5/p35 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 101nMAssay Description:Inhibition of recombinant FLT3 D835Y mutant (unknown origin) in presence of ATP by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 101nMAssay Description:Inhibition of FLT3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of GSK3beta (unknown origin) in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of GSK3beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of LCK (unknown origin) in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibition of SGK (unknown origin) in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 440nMAssay Description:Inhibition of CDK2/cyclin A (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase tousled-like 2(Human)
University of North Carolina at Chapel Hill
Curated by ChEMBL
University of North Carolina at Chapel Hill
Curated by ChEMBL
Affinity DataIC50: 440nMAssay Description:Inhibition of TLK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 590nMAssay Description:Inhibition of GST-fused human CDK2/cyclin A expressed in Escherichia coli using histone H1 as substrate in presence of [33p]-gamma ATP and MgATPMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase 17B(Human)
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Affinity DataIC50: 710nMAssay Description:Inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by ADP-Glo kinase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of FGFR1 (unknown origin)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D1(Human)
Zunyi Medical University
Curated by ChEMBL
Zunyi Medical University
Curated by ChEMBL
Affinity DataIC50: 3.33E+3nMAssay Description:Inhibition of CDK4/cyclin D1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.30E+3nMAssay Description:Inhibition of IGF1R (unknown origin) by ADP-Glo assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)