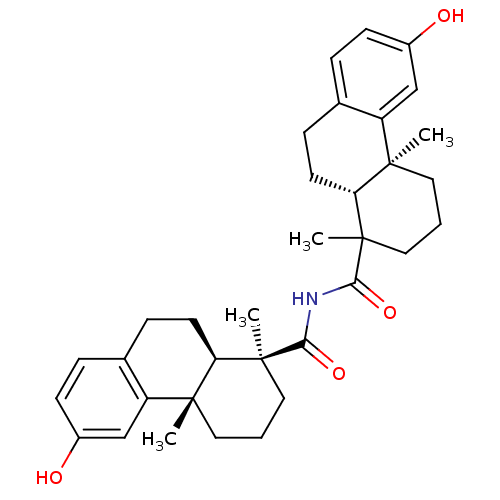
BDBM50167697 (4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-phenanthrene-1-carboxylic acid ((1S,4aS,10aR)-6-hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-phenanthrene-1-carbonyl)-amide::(4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-phenanthrene-1-carboxylic acid ((1S,4aS,10aR)-6-hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-phenanthrene-1-carbonyl)-amide::CHEMBL365544
SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21
InChI Key InChIKey=ASTLYMNMTDERKI-IMUKZWIGSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50167697
Found 12 hits for monomerid = 50167697
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibitory concentration in LXRSPA beta binding assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: 1nMAssay Description:Effective concentration in recombinant human LXRalpha ligand binding domain in homogeneous time-resolved fluorescence assay More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: <3nMAssay Description:Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRalpha receptorMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: 1nMAssay Description:Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: <3nMAssay Description:Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRbeta receptorMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: 1nMAssay Description:Effective concentration for cofactor association with recombinant liver X receptor-alphaMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: 1nMAssay Description:Effective concentration for cofactor association with recombinant liver X receptor-betaMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: <3nMAssay Description:Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibitory concentration in LXRSPA alpha binding assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: <3nMAssay Description:Effective concentration against liver X receptor-beta in HEK293 cell transactivation assayMore data for this Ligand-Target Pair