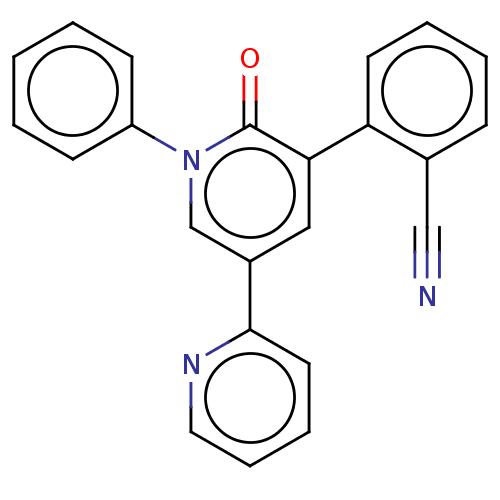
BDBM50184410 CHEBI:71013::E-2007::E2007::ER-155055-90::Fycompa::Inhibitor 1 of SARS-CoV-2 Mpro::Perampanel::Perampanel, 1::US20240092759, Compound Perampanel
SMILES c1ccc(cc1)N2C=C(C=C(C2=O)c3ccccc3C#N)c4ccccn4
InChI Key InChIKey=PRMWGUBFXWROHD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50184410
Found 11 hits for monomerid = 50184410
Affinity DataIC50: 243nMAssay Description:Antagonist activity at human iGluA1 receptor flop isoform expressed in CHO-S cells coexpressing TARP gamma-3 assessed as inhibition of glutamate-indu...More data for this Ligand-Target Pair
Affinity DataIC50: 485nMAssay Description:Antagonist activity at human iGluA1 receptor flop isoform expressed in CHO-S cells coexpressing TARP gamma-2 assessed as inhibition of glutamate-indu...More data for this Ligand-Target Pair
Affinity DataIC50: 623nMAssay Description:Antagonist activity at human iGluA1 receptor flop isoform expressed in CHO-S cells coexpressing TARP gamma-4 assessed as inhibition of glutamate-indu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.18E+3nMAssay Description:Antagonist activity at human iGluA1 receptor flop isoform expressed in CHO-S cells coexpressing TARP gamma-8 and human EAAT3 assessed as inhibition o...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Blockade of whole cell current in GluA3i-G (unknown origin) transfected in HEK293 cells measured at -60 mV in presence of glutamate by whole cell pat...More data for this Ligand-Target Pair
Affinity DataIC50: 6.51E+3nMAssay Description:Antagonist activity at human iGluA1 receptor flip isoform expressed in CHO-S cells assessed as inhibition of glutamate-induced increase in intracellu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of proteolytic activity was tested using recombinant SARS-Cov-2 Mpro, as described herein in the Experimental section. For the kinetic ass...More data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(2019-nCoV)
Shaanxi University of Science & Technology
Curated by ChEMBL
Shaanxi University of Science & Technology
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of SARS-CoV-2 recombinant main proteaseMore data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(2019-nCoV)
Shaanxi University of Science & Technology
Curated by ChEMBL
Shaanxi University of Science & Technology
Curated by ChEMBL
Affinity DataIC50: 1.75E+5nMAssay Description:Purified protein is diluted in reaction buffer to 100 nM in an opaque 96-well plate. The protein is incubated with or without compound in DMSO at dif...More data for this Ligand-Target Pair
Affinity DataIC50: 1.75E+5nMAssay Description:Inhibition of proteolytic activity was tested using recombinant SARS-Cov-2 Mpro, as described herein in the Experimental section. For the kinetic ass...More data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(2019-nCoV)
Shaanxi University of Science & Technology
Curated by ChEMBL
Shaanxi University of Science & Technology
Curated by ChEMBL
Affinity DataIC50: 1.75E+5nMAssay Description:Inhibition of proteolytic activity was tested using recombinant SARS-CoV-2Mpro, which was expressed and purified as previously described.8,12 For the...More data for this Ligand-Target Pair