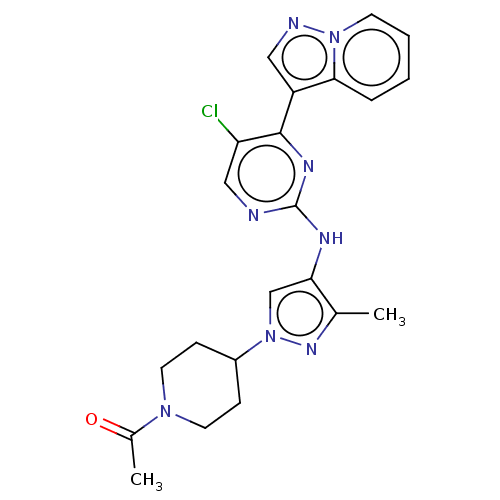
BDBM50184475 CHEMBL3822989
SMILES CC(=O)N1CCC(CC1)n1cc(Nc2ncc(Cl)c(n2)-c2cnn3ccccc23)c(C)n1
InChI Key InChIKey=HIMUEIQDEDWCOT-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50184475
Found 4 hits for monomerid = 50184475
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human IGF-1R using fluorescent labeled FL-KKSRGDYMTMQIG-CONH2 as substrate after 1 hr 50 minsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of human ERG expressed in CHOK1 cells after 6 mins by electrophysiology assayMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of IGF1-induced human IGF1R autophosphorylation expressed in IGF-1R knock-out mouse fibroblastsMore data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Inhibition of CDK2 (unknown origin)More data for this Ligand-Target Pair