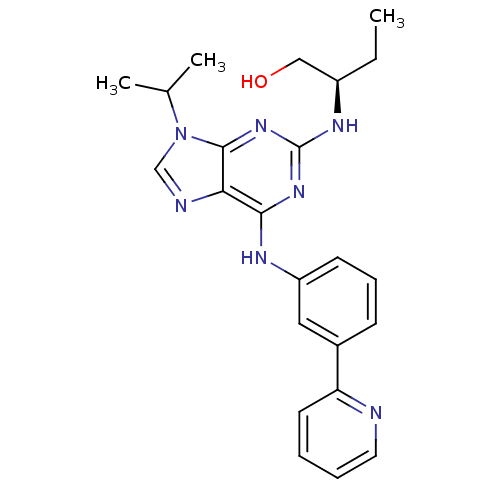
BDBM50244787 (R)-2-(1-Hydroxybut-2-ylamino)-6-[3-(2-pyridyl)phenylamino]-9-isopropylpurine::CHEMBL215919
SMILES CC[C@H](CO)Nc1nc(Nc2cccc(c2)-c2ccccn2)c2ncn(C(C)C)c2n1
InChI Key InChIKey=LWANFAFTTOKZAX-QGZVFWFLSA-N
Data 15 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 50244787
Found 15 hits for monomerid = 50244787
TargetGlycogen synthase kinase-3 beta/[Tau protein] kinase(Sus scrofa)
Universite Paris-Descartes
Curated by ChEMBL
Universite Paris-Descartes
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of pig brain GSK3alpha/betaMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of GSK3-betaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
University Of Paris
Curated by ChEMBL
University Of Paris
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation countingMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
University Of Paris
Curated by ChEMBL
University Of Paris
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of CDK2/cyclin B (unknown origin) after 30 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of CK1 using KRRRALS(p)VASLPGL as substrate after 40 mins by scintillation counterMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D1(Homo sapiens (Human))
Palack£
Curated by ChEMBL
Palack£
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of GST-tagged CDK4/cyclin D1 (unknown origin) expressed in Baculovirus infected Sf9 cells using RPPTLSPIPHIPR peptide as substrate in pres...More data for this Ligand-Target Pair
Affinity DataIC50: 290nMAssay Description:Inhibition of GST-tagged CDK2/cyclin A2 (unknown origin) expressed in Escherichia coli using histone H1 as substrate in presence of [gamma-33P]-ATP b...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
University Of Paris
Curated by ChEMBL
University Of Paris
Curated by ChEMBL
Affinity DataIC50: 215nMAssay Description:Inhibition of GST-tagged CDK5/p25 (unknown origin) expressed in Baculovirus infected Sf9 cells using histone H1 as substrate as substrate in presence...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of GST-tagged CDK9/CyclinT1 (unknown origin) expressed in Baculovirus infected Sf9 cells using YSPTSPS-2 KK peptide as substrate as substr...More data for this Ligand-Target Pair
TargetCDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7(Homo sapiens (Human))
Palack£
Curated by ChEMBL
Palack£
Curated by ChEMBL
Affinity DataIC50: 822nMAssay Description:Inhibition of GST-tagged CDK7/cyclinH/MAT1 (unknown origin) expressed in Baculovirus infected Sf9 cells using YSPTSPS-2 KK peptide as substrate as su...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
University Of Paris
Curated by ChEMBL
University Of Paris
Curated by ChEMBL
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of His-tagged CDK1/cyclin B1 (unknown origin) expressed in Baculovirus infected Sf9 cells using histone H1 as substrate in presence of [ga...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Palack£
Curated by ChEMBL
Palack£
Curated by ChEMBL
Affinity DataIC50: 93nMAssay Description:Inhibition of His-tagged CDK2/cyclin E (unknown origin) expressed in Baculovirus infected Sf9 cells using histone H1 as substrate in presence of [gam...More data for this Ligand-Target Pair