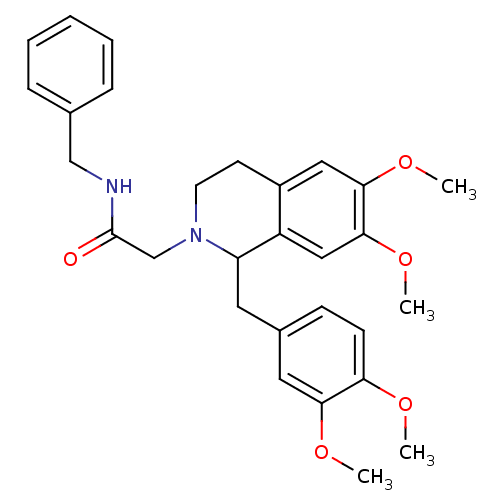
BDBM50292928 (+/-)-N-benzyl-2-(1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)acetamide::CHEMBL490489
SMILES COc1ccc(CC2N(CC(=O)NCc3ccccc3)CCc3cc(OC)c(OC)cc23)cc1OC
InChI Key InChIKey=FYSUZYYFUUADES-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50292928
Found 4 hits for monomerid = 50292928
Affinity DataIC50: 119nMAssay Description:Antagonist activity at OX1R (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 119nMAssay Description:Inhibition of OX1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 8.15E+3nMAssay Description:Antagonist activity at OX2R (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.15E+3nMAssay Description:Inhibition of OX2 receptorMore data for this Ligand-Target Pair