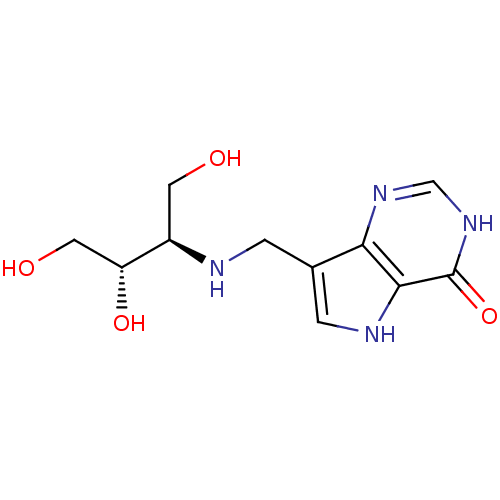
BDBM50293091 7-({[(1R,2S)-2,3-DIHYDROXY-1-(HYDROXYMETHYL)PROPYL]AMINO}METHYL)-3,5-DIHYDRO-4H-PYRROLO[3,2-D]PYRIMIDIN-4-ONE::7-({[(2R,3S)-1,3,4-Trihydroxybutan-2-yl]amino}methyl)-3,5-dihydro-4H-pyrrolo[3,2-d]pyrimidin-4-one::CHEMBL475537
SMILES OC[C@@H](O)[C@@H](CO)NCc1c[nH]c2c1nc[nH]c2=O
InChI Key InChIKey=CGYSFECPLYEOMH-HTQZYQBOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50293091
Found 4 hits for monomerid = 50293091
Affinity DataKi: 0.00900nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKd: 55nMAssay Description:Binding affinity to Plasmodium falciparum His6-tagged PNP assessed as reduction in uric acid formation using inosine as substrateMore data for this Ligand-Target Pair
Affinity DataKd: 0.00860nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 55nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)