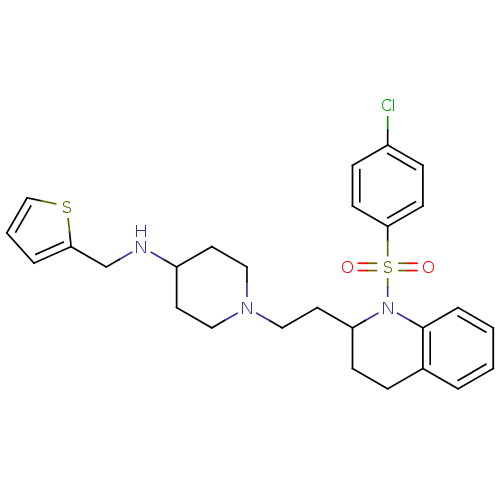
BDBM50299338 1-(2-(1-(4-chlorophenylsulfonyl)-1,2,3,4-tetrahydroquinolin-2-yl)ethyl)-N-(thiophen-2-ylmethyl)piperidin-4-amine::CHEMBL577599
SMILES Clc1ccc(cc1)S(=O)(=O)N1C(CCN2CCC(CC2)NCc2cccs2)CCc2ccccc12
InChI Key InChIKey=YWKPGWQHNFOGPP-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50299338
Found 3 hits for monomerid = 50299338
TargetVasopressin V1b receptor(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 7.20E+3nMAssay Description:Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 9.59E+3nMAssay Description:Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counterMore data for this Ligand-Target Pair
TargetVasopressin V1a receptor(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]Arg8-vasopressin from human vasopressin V1a receptor expressed in CHO-K1 cells by Packard Topcount scintillation counterMore data for this Ligand-Target Pair