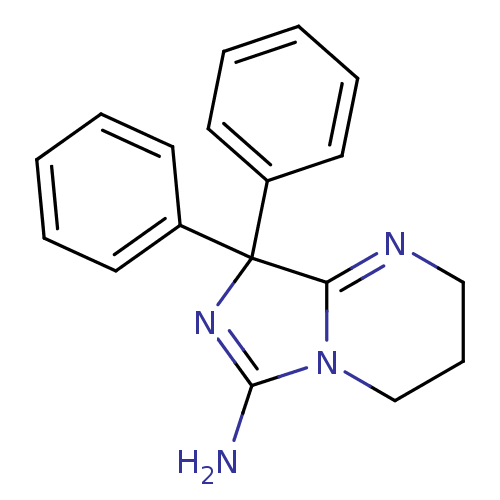
BDBM50300710 8,8-diphenyl-2,3,4,8-tetrahydroimidazo[1,5-a]pyrimidin-6-amine::CHEMBL582044
SMILES NC1=NC(C2=NCCCN12)(c1ccccc1)c1ccccc1
InChI Key InChIKey=UFWSJOVQEPTPNE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 50300710
Found 15 hits for monomerid = 50300710
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of human BACE1 by FRET based peptide cleavage assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.83E+4nMAssay Description:Inhibition of human BACE2 by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of human BACE1 by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of human BACE1 by FRETMore data for this Ligand-Target Pair
Affinity DataEC50: 1.60E+4nMAssay Description:Inhibition of BACE1 expressed in CHO cells co-expressing human wild type APP protein by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of human BACE1 by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of BACE1 by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of human BACE1 by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.83E+4nMAssay Description:Inhibition of human BACE2 by FRETMore data for this Ligand-Target Pair
Affinity DataEC50: 1.60E+4nMAssay Description:Inhibition of human recombinant APP expressed in CHO-K1 cells assessed as reduction of amyloid beta level by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of human BACE2 by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.83E+4nMAssay Description:Inhibition of BACE2 by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of cathepsin D by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human cathepsin D by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:Inhibition of BACE1 expressed in CHOK1 cells coexpressing human recombinant wild type APP assessed as blockade of amyloid beta production by ELISAMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)