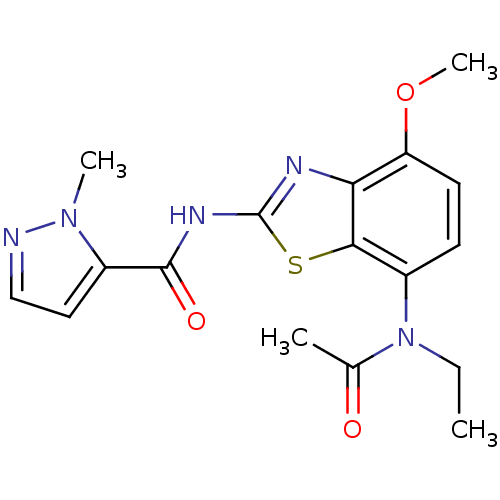
BDBM50321518 CHEMBL1170367::N-(7-(N-ethylacetamido)-4-methoxybenzo[d]thiazol-2-yl)-1-methyl-1H-pyrazole-5-carboxamide
SMILES CCN(C(C)=O)c1ccc(OC)c2nc(NC(=O)c3ccnn3C)sc12
InChI Key InChIKey=CPBGMRFHFZVCLS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50321518
Found 14 hits for monomerid = 50321518
Affinity DataKi: 8nMAssay Description:Displacement of [3H]ZM241385 from human adenosine A2B receptor expressed in CHO cells after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Displacement of [3H]ZM241385 from human adenosine A2B receptor expressed in CHO cells after 1 hr by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:Displacement of [3H]ZM241385 from human adenosine A2A receptor after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in CHO-K1 cells after 1 hr by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Binding affinity to adenosine A3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Displacement of [3H]-DPCPX from human adenosine A1 receptor after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO-K1 cells after 1 hr by scintillation countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Antagonist activity at human adenosine A2B receptor expressed in CHO cells assessed as inhibition of adenosine-induced cAMP productionMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of human adenosine A2B receptor expressed in CHO cells assessed as decrease in cellular cAMP level after 20 to 25 minsMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair