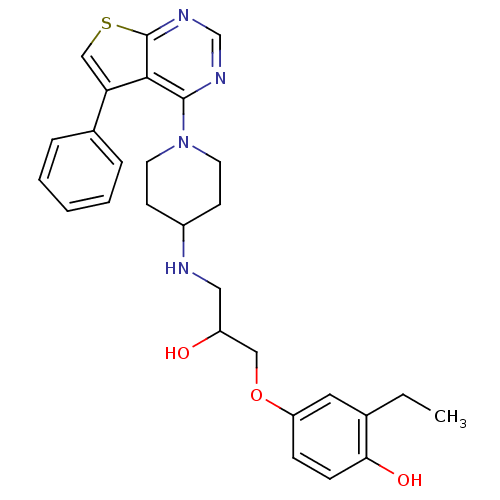
BDBM50328298 2-ethyl-4-(2-hydroxy-3-(1-(5-phenylthieno[2,3-d]pyrimidin-4-yl)piperidin-4-ylamino)propoxy)phenol::CHEMBL1257797
SMILES CCc1cc(OCC(O)CNC2CCN(CC2)c2ncnc3scc(-c4ccccc4)c23)ccc1O
InChI Key InChIKey=HNMSNLOAZLYBDB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50328298
Found 4 hits for monomerid = 50328298
Affinity DataKi: 9nMAssay Description:Displacement of [125I]-cyanopindolol from human adrenergic beta3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [125I]-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 287nMAssay Description:Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as cyclic AMP formation by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.140nMAssay Description:Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as cyclic AMP formation by HTRF assayMore data for this Ligand-Target Pair