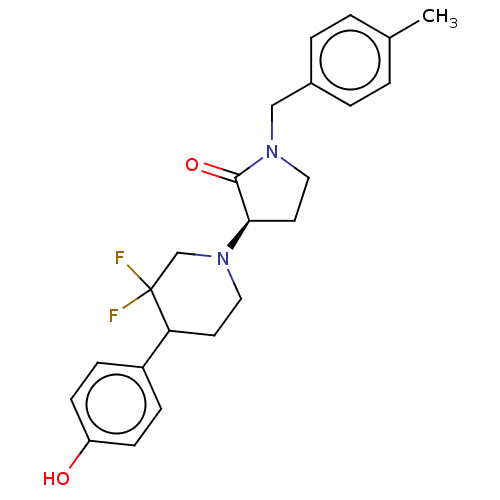
BDBM50330409 CHEMBL4168402
SMILES Cc1ccc(CN2CC[C@@H](N3CCC(c4ccc(O)cc4)C(F)(F)C3)C2=O)cc1
InChI Key InChIKey=HKJQOALDDUBRBV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50330409
Found 2 hits for monomerid = 50330409
TargetGlutamate receptor ionotropic, NMDA 2B(Rat)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataKi: 7.70nMAssay Description:Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology...More data for this Ligand-Target Pair