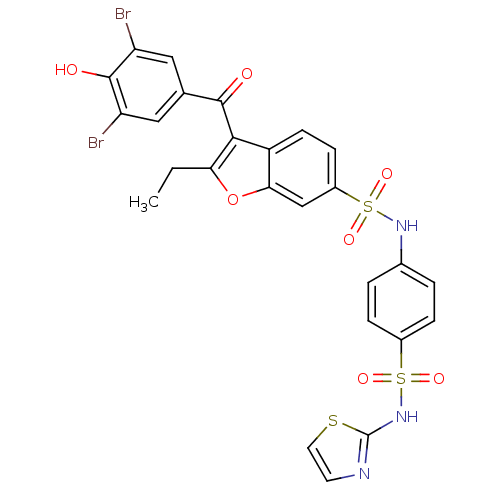
BDBM50341989 3-(3,5-DIBROMO-4-HYDROXY-BENZOYL)-2-ETHYL-BENZOFURAN-6-SULFONIC ACID [4-(THIAZOL-2-YLSULFAMOYL)-PHENYL]-AMIDE::3-(3,5-dibromo-4-hydroxybenzoyl)-2-ethyl-N-(4-(N-thiazol-2-ylsulfamoyl)phenyl)benzofuran-6-sulfonamide::CHEMBL1232829
SMILES CCc1c(c2ccc(cc2o1)S(=O)(=O)Nc3ccc(cc3)S(=O)(=O)Nc4nccs4)C(=O)c5cc(c(c(c5)Br)O)Br
InChI Key InChIKey=SXKBTDJJEQQEGE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50341989
Found 8 hits for monomerid = 50341989
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Northeastern University
Curated by ChEMBL
Northeastern University
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of GST-tagged PTP1B (1 to 321 residues) (unknown origin) using p-NPP as substrate preincubated for 10 mins followed by substrate addition ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
Leibniz-Forschungsinstitut F�R Molekulare Pharmakologie (Fmp)
Curated by ChEMBL
Leibniz-Forschungsinstitut F�R Molekulare Pharmakologie (Fmp)
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Northeastern University
Curated by ChEMBL
Northeastern University
Curated by ChEMBL
Affinity DataIC50: 6.81E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using nitrophenyl phosphate as substrate after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Northeastern University
Curated by ChEMBL
Northeastern University
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of recombinant human PTP1B assessed as inhibition of hydrolysis of pNPP by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Northeastern University
Curated by ChEMBL
Northeastern University
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of PTP1B (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Northeastern University
Curated by ChEMBL
Northeastern University
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Northeastern University
Curated by ChEMBL
Northeastern University
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Allosteric inhibition of PTP1B (unknown origin) using pNPP as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of RUVBL1 (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)