BDBM50355393 BGJ398::CHEMBL1834657::US9434697, BGJ398::US9730931, BGJ398
SMILES CCN1CCN(CC1)c2ccc(cc2)Nc3cc(ncn3)N(C)C(=O)Nc4c(c(cc(c4Cl)OC)OC)Cl
InChI Key InChIKey=QADPYRIHXKWUSV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 138 hits for monomerid = 50355393
Found 138 hits for monomerid = 50355393
Affinity DataIC50: 0.5nMAssay Description:Inhibition of wild-type FGFR1 (unknown origin) by radiometric kinase activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of wild-type FGFR2 (unknown origin) by radiometric kinase activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of FGFR3 K650E mutant (unknown origin) by radiometric kinase activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:Inhibition of N-terminal GST-tagged human FGFR1 cytoplasmic domain (398-822 AA) expressed in baculovirus using FAM-labelled peptide as substrate pre-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:Inhibition of N-terminal GST-tagged human FGFR1 cytoplasmic domain (398-822 AA) expressed in baculovirus using FAM-labelled peptide as substrate pre-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMAssay Description:Inhibition of wild-type FGFR3 (unknown origin) by radiometric kinase activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMAssay Description:Inhibition of FGFR2 N549H mutant (unknown origin) by radiometric kinase activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of FGFR3 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of recombinant FGFR1More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of FGFR1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant FGFR1 (unknown origin) using peptidic substrates in presence of ATP by Kinase-Glo luminescent kinase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of FGFR3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Recombinant FGFR1 (2.5 nM), or FGFR4 (12 nM) was prepared as a mixture with substrate KKKSPGEYVNIEFG (SEQ ID NO:1) (20 μM, FGFR1 substrate); Pol...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Recombinant FGFR1 (2.5 nM), FGFR2 (1 nM), FGFR3 (5 nM), or FGFR4 (12 nM) (Invitrogen ) was prepared as a mixture with substrate KKKSPGEYVNIEFG (SEQ I...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant GST fused FGFR3 (unknown origin) using poly(EY) 4:1 as substrate in presence of [gamma-32P]ATP after 10 mins by scintillati...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant FGFR2 (unknown origin) using peptidic substrates in presence of ATP by Kinase-Glo luminescent kinase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:Recombinant FGFR1 (2.5 nM), or FGFR4 (12 nM) was prepared as a mixture with substrate KKKSPGEYVNIEFG (SEQ ID NO:1) (20 μM, FGFR1 substrate); Pol...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant FGFR3More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of recombinant FGFR2More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of FGFR2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of FGFR3-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of FGFR2 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of recombinant human FGFR1 using biotin-EQEDEPEGDYFEWLE-amide as substrate preincubated for 5 to 10 mins followed by substrate addition an...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Inhibition of N-terminal His-Avi tagged recombinant human FGFR3 (447 to 761 residues) expressed in an Sf9 infected baculovirus expression system usin...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Inhibition of FGFR1-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:Inhibition of wild type FGFR1 expressed in HEK293 cells assessed as inhibition of autophosphorylation of tyrosine residue after 40 mins by ELISA assa...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:Inhibition of wild type FGFR2 expressed in HEK293 cells assessed as inhibition of autophosphorylation of tyrosine residue after 40 mins by ELISA assa...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Recombinant FGFR1 (2.5 nM), or FGFR4 (12 nM) was prepared as a mixture with substrate KKKSPGEYVNIEFG (SEQ ID NO:1) (20 μM, FGFR1 substrate); Pol...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Recombinant FGFR1 (2.5 nM), FGFR2 (1 nM), FGFR3 (5 nM), or FGFR4 (12 nM) (Invitrogen ) was prepared as a mixture with substrate KKKSPGEYVNIEFG (SEQ I...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.5 T: 2°CAssay Description:Recombinant FGFR1 (2.5 nM), or FGFR4 (12 nM) was prepared as a mixture with substrate KKKSPGEYVNIEFG (SEQ ID NO:1) (20 μM, FGFR1 substrate); Pol...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of FGFR3 K650M mutant (unknown origin) by radiometric kinase activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of FGFR4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant FGFR4More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of wild-type FGFR4 (unknown origin) by radiometric kinase activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant FGFR4 (unknown origin) using peptidic substrates in presence of ATP by Kinase-Glo luminescent kinase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 71nMAssay Description:Inhibition of recombinant non-phosphorylated FGFR4 kinase domain (442 to 753) (unknown origin) expressed in Sf9 insect cells using 5-Fluo-Ahx-KKKKEEI...More data for this Ligand-Target Pair
Affinity DataIC50: 115nMAssay Description:Inhibition of recombinant human FGFR4 (460 to 802 residues) using biotin-EQEDEPEGDYFEWLE-amide as substrate preincubated for 5 to 10 mins followed by...More data for this Ligand-Target Pair
Affinity DataIC50: 168nMAssay Description:Inhibition of wild type FGFR4 expressed in HEK293 cells assessed as inhibition of autophosphorylation of tyrosine residue after 40 mins by ELISA assa...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Human)
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of recombinant VEGFR2More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of recombinant LYN kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 352nMAssay Description:Inhibition of recombinant human FGFR3 V555M mutant using biotin-EQEDEPEGDYFEWLE-amide as substrate preincubated for 5 to 10 mins followed by substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 506nMAssay Description:Inhibition of FGFR3 V555M mutant (unknown origin) by radiometric kinase activity assayMore data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 4(Mouse)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 541nMAssay Description:Inhibition of FGFR4 in mouse BAF3 cells assessed as decrease in FGFR4 phosphorylation incubated for 40 minsMore data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 4(Mouse)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 581nMAssay Description:Inhibition of FGFR4 in mouse BAF3 cells assessed as reduction in cell viability incubated for 2 days by cell proliferation assayMore data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Human)
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 750nMAssay Description:Inhibition of recombinant KIT kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 759nMAssay Description:Inhibition of FGFR1 V561M mutant (unknown origin) by radiometric kinase activity assayMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Human)
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 938nMAssay Description:Inhibition of VEGFR2 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of recombinant YES kinaseMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Human)
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.45E+3nMAssay Description:Inhibition of VEGFR2-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1(Human)
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.51E+3nMAssay Description:Inhibition of VEGFR1 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
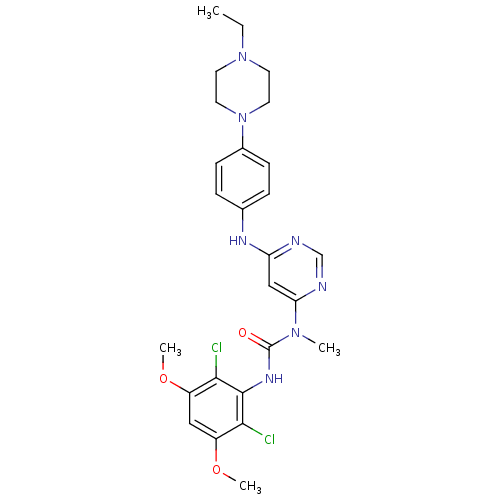
 3D Structure (crystal)
3D Structure (crystal)