BDBM50355393 BGJ398::CHEMBL1834657::US9434697, BGJ398::US9730931, BGJ398
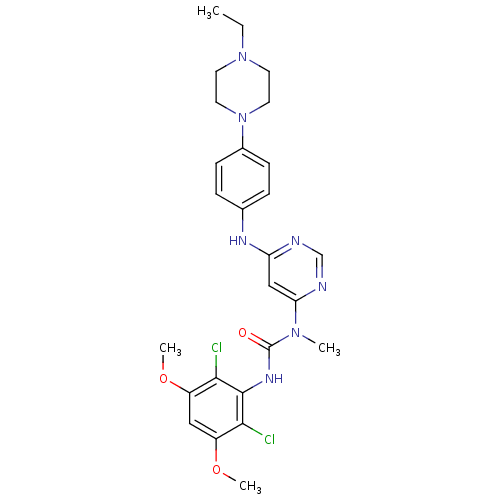
SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1
InChI Key InChIKey=QADPYRIHXKWUSV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 138 hits for monomerid = 50355393
Found 138 hits for monomerid = 50355393
Affinity DataIC50: 13nMpH: 7.5 T: 2°CAssay Description:Recombinant FGFR1 (2.5 nM), or FGFR4 (12 nM) was prepared as a mixture with substrate KKKSPGEYVNIEFG (SEQ ID NO:1) (20 μM, FGFR1 substrate); Pol...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:Recombinant FGFR1 (2.5 nM), or FGFR4 (12 nM) was prepared as a mixture with substrate KKKSPGEYVNIEFG (SEQ ID NO:1) (20 μM, FGFR1 substrate); Pol...More data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit beta(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant CAMK2More data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of KIT juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetMacrophage-stimulating protein receptor(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant MST1RMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 3(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.07E+3nMAssay Description:Inhibition of VEGFR3 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 3(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of FGFR3-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant PKCalphaMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.45E+3nMAssay Description:Inhibition of VEGFR2-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetInsulin receptor(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.37E+3nMAssay Description:Inhibition of INSR-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
Affinity DataIC50: 168nMAssay Description:Inhibition of wild type FGFR4 expressed in HEK293 cells assessed as inhibition of autophosphorylation of tyrosine residue after 40 mins by ELISA assa...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.08E+3nMAssay Description:Inhibition of ALK juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 938nMAssay Description:Inhibition of VEGFR2 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.97E+3nMAssay Description:Inhibition of SRC-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant PDGFRalphaMore data for this Ligand-Target Pair
TargetMacrophage-stimulating protein receptor(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.89E+3nMAssay Description:Inhibition of RON-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant RETMore data for this Ligand-Target Pair
TargetInsulin receptor(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant INSRMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant CDK1More data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 2(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of FGFR2 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetRho-associated protein kinase 2(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant ROCK2More data for this Ligand-Target Pair
TargetTyrosine-protein kinase SYK(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.36E+3nMAssay Description:Inhibition of SYK-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase JAK2(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant JAK2More data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 3(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of FGFR3 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant METMore data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 2(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of recombinant FGFR2More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lyn(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.02E+3nMAssay Description:Inhibition of LYN-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Inhibition of FGFR1-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of recombinant FGFR1More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of TYK2-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 using DBF as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 using BFC as substrateMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant SRCMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase SYK(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant SYKMore data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant TYK2More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant VPS34 by luminescent kinase assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant WNK1More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ZAP-70(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant ZAP70More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.25E+3nMAssay Description:Inhibition of FLT3-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetAngiopoietin-1 receptor(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.05E+3nMAssay Description:Inhibition of TIE2-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetEphrin type-B receptor 1(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.04E+3nMAssay Description:Inhibition of EPHB1-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.72E+3nMAssay Description:Inhibition of PDGFRalpha-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.01E+3nMAssay Description:Inhibition of MET-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase JAK2(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.88E+3nMAssay Description:Inhibition of JAK2-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase ROS(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.26E+3nMAssay Description:Inhibition of ROS-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetBDNF/NT-3 growth factors receptor(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.14E+3nMAssay Description:Inhibition of TRKB juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetCytoplasmic tyrosine-protein kinase BMX(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.41E+3nMAssay Description:Inhibition of BMX-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase constructMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C19 using CEC as substrateMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of JNK2More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >9.00E+3nMAssay Description:Inhibition of PI4Kbeta by luminescent kinase assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)