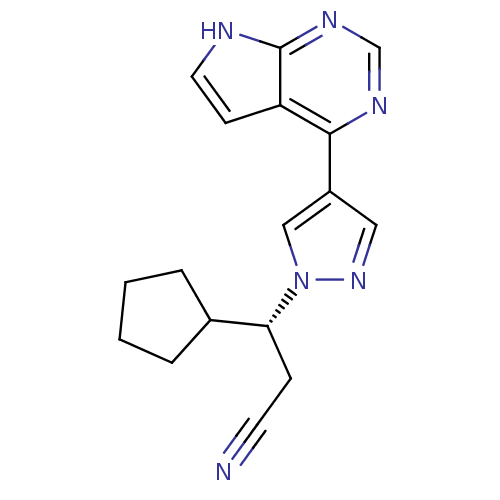
BDBM50355501 INCB-018424::RUXOLITINIB::RUXOLITINIB PHOSPHATE::US10112907, Example 00016::US10766894, Compound TABLE 1.1::US10875847, Compound JAKAFI::US11203595, TABLE 1.1::US11279703, TABLE 6.147::US20240140952, Compound Ruxolitinib
SMILES c1c[nH]c2c1c(ncn2)c3cnn(c3)[C@H](CC#N)C4CCCC4
InChI Key InChIKey=HFNKQEVNSGCOJV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 634 hits for monomerid = 50355501
Found 634 hits for monomerid = 50355501
Affinity DataIC50: 0.0360nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.0360nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKd: 0.0360nMAssay Description:Binding constant for JAK2(JH1domain-catalytic) kinase domainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0560nMAssay Description:Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0560nMAssay Description:Inhibition of human JAK2 using poly[Glu:Tyr] as substrate in presence of [gamma-33P]-ATPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Inhibition of purified JAK1 incubated for 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Inhibition of JAK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Inhibition of JAK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of JAK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of human JAK2 (828-1132) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.120nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of human JAK1 (837-1142) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human recombinant JAK2More data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:Inhibition of purified JAK2 incubated for 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human JAK2 by radiometric assayMore data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of TYK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Inhibition of JAK1 (unknown origin)More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of TYK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m...More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 0.550nMAssay Description:Inhibition of purified TYK2 incubated for 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of JAK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human recombinant N-terminal hexahistidine tagged JAK2 JH1 catalytic domain (835 to 1132 residues) expressed in baculovirus infected Sf...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.720nMAssay Description:Inhibition of TYK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKd: 0.800nMAssay Description:Binding affinity to recombinant human JAK2 kinase domain by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of JAK1 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas...More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKd: 0.900nMAssay Description:Binding constant for TYK2(JH1domain-catalytic) kinase domainMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma33P]-ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.5Assay Description:A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J...More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKd: 1.10nMAssay Description:Binding affinity to recombinant human TYK2 kinase domain by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of JAK1 (unknown origin) preincubated for 60 mins followed by reagent A addition and measured after 60 mins in presence of ATP by using mi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human recombinant JAK1More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of JAK3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of JAK3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKd: 2nMAssay Description:Binding constant for JAK3(JH1domain-catalytic) kinase domainMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.5Assay Description:A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of N-terminal epitope tagged recombinant human JAK2 (828 to 1132 residues) expressed in baculovirus infected Sf21 cells using EQEDEPEGDYFE...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of JAK 2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of human JAK2 (828 to 1132 residues) expressed in baculovirus infected Sf9 insect cells using EQEDEPEGDYFEWLE as substrate after 1 hr by f...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)