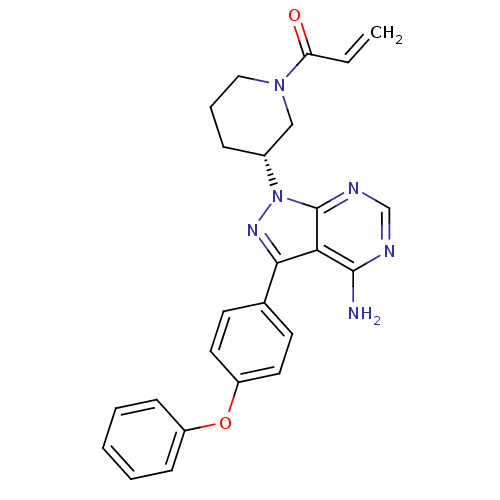
BDBM50357312 IBRUTINIB::PCI-32765::US10124003, Ref. Ex. Compound 1::US10711006, Compound Ibrutinib::US10793575, Example ibrutinib::US10835536, Ref. Ex. Comp 1::US10919899, Ibrutinib::US11078206, Example Ibrutinib::US11186578, Example Ibrutinib::US11339167, Example Ibrutinib::US11407754, Example Ibrutinib::US20230364079, Example Ibrutinib::US20240059694, Compound Ibrutinib::US20240139326, Compound Ibrutinib::US9108973, Ref 1::US9181263, 1::US9278100, 1
SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C
InChI Key InChIKey=XYFPWWZEPKGCCK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 442 hits for monomerid = 50357312
Found 442 hits for monomerid = 50357312
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.0800nMAssay Description:Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of recombinant full length human His-tagged BLK cytoplasmic domain expressed in baculovirus expression system using tyrosine-1 peptide as ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of recombinant BLK (unknown origin) preincubated for 1 hr in presence of ATP by Z-Lyte assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Covalent inhibition of full length N-terminal GST tagged BTK (2 to 659 residues) (unknown origin) using AQT0101 as substrate preincubated for 60 mins...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of BLK (unknown origin) incubated for 1 hr in presence of ATP by Z'LYTE assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of BLK (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition by filter binding methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:BTK kinase domain was expressed and purified as previously reported65. Binding experiments were performed in Tris 20 mM pH=8, 50 mM NaCl, and 1 mM DT...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human full length BTK using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by microfluid mobility shift assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of BTK (unknown origin) measured by ADP-Glo assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.210nMAssay Description:Inhibition of BTK (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.210nMAssay Description:The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In...More data for this Ligand-Target Pair
Affinity DataIC50: 0.230nMAssay Description:Inhibition of human BLK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.230nMAssay Description:Inhibition of human BLK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataKi: 0.240nMAssay Description:Binding affinity to BTK (unknown origin) assessed as inhibition constant measured after 60 mins incubation by microtiter-plate readerMore data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of human HER4 using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of human HER4 using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 ...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of recombinant human N-terminal His-tagged BTK expressed in baculovirus infected sf9 cells using poly(4:1 Glu,Tyr) as substrate by ADP-Glo...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of recombinant full-length N-terminal His-tagged human BTK expressed in baculovirus infected Sf9 insect cells using Poly(4:1 Glu,Tyr) pept...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of recombinant human His-tagged full length BTK expressed in baculovirus expression system using Ulight-Poly GT as substrate incubated for...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.330nMAssay Description:Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:Inhibition of human recombinant full-length N-terminal His-tagged BTK expressed in baculovirus infected Sf9 insect cells after 60 mins by ADP-Glo kin...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:Inhibition of N-terminal His-tagged full length human recombinant BTK expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Glu, Tyr) pe...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:Inhibition of human recombinant full-length N-terminal His-tagged BTK expressed in baculovirus infected Sf9 insect cells measured after 60 mins by AD...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of full-length N-terminal GST-tagged BMX (1 to 675 residues) (unknown origin) expressed in Sf21 insect cells using NH2-ETVYSEVRK-biotin as...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of full length recombinant human N-terminal His-tagged BTK expressed in baculovirus infected Sf9 cells using poly (4:1 Glu, Tyr) peptide a...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human BTK using Poly(Glu, Tyr) 4:1 as substrate after 1 hr by ELISAMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.420nMAssay Description:Inhibition of BTK (unknown origin) by lanthascreen Tb kinase activity assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.430nMAssay Description:Inhibition of His-tagged recombinant human His-tagged full length BTK expressed in baculovirus expression system using Tyr01 peptide as substrate pre...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.460nMAssay Description:Inhibition of BTK (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.460nMAssay Description:Inhibition of human BTK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.460nMAssay Description:Inhibition of human BTK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.480nMAssay Description:Inhibition of BTK (unknown origin) measured after 60 mins incubation by microtiter-plate readerMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of recombinant human TEC using Poly(Glu, Tyr) 4:1 as substrate after 1 hr by ELISAMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of BTK (unknown origin) One-hour enzymatic assay without pre-incubationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Irreversible inhibition of recombinant BTK (unknown origin) incubated for 1 hr by FRET assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of BTK (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Irreversible inhibition of recombinant human BTKMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of BTK (unknown origin) using TK as substrate incubated for 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of BTK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition...More data for this Ligand-Target Pair