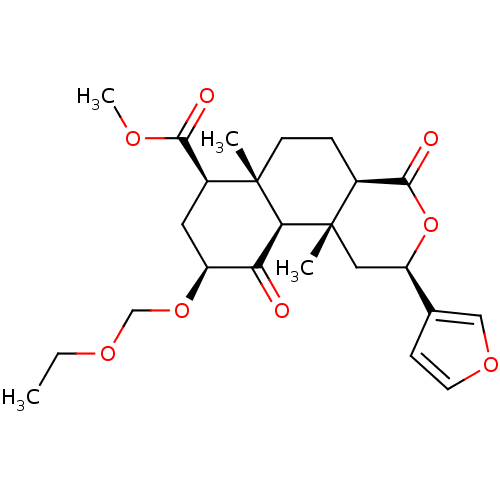
BDBM50362540 CHEMBL1941031
SMILES CCOCO[C@H]1C[C@@H](C(=O)OC)[C@]2(C)CC[C@H]3C(=O)O[C@H](C[C@]3(C)[C@H]2C1=O)c1ccoc1
InChI Key InChIKey=ICVTXAUKIHJDGV-BXEHRHDKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50362540
Found 3 hits for monomerid = 50362540
Affinity DataKi: 2.5nMAssay Description:Displacement of [3H]diprenorphine from human KOPR expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 123nMAssay Description:Agonist activity at human KOPR expressed in U2OS cells assessed as beta-arrestin2 recruitment after 90 mins by DiscoveRx PathHunter assayMore data for this Ligand-Target Pair
Affinity DataEC50: 9.5nMAssay Description:Agonist activity at human KOPR expressed in CHO cells assessed as [35S]GTPgammaS binding after 60 mins by liquid scintillation countingMore data for this Ligand-Target Pair