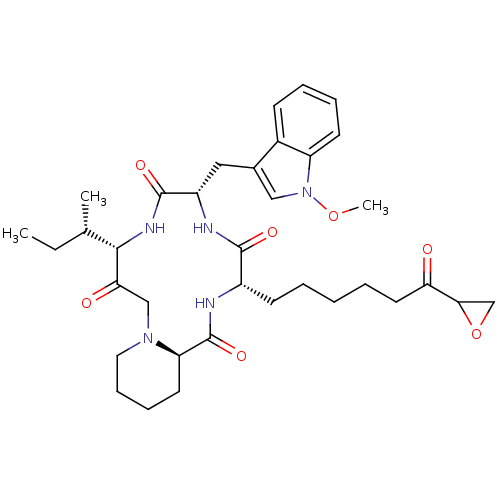
BDBM50366693 CHEMBL1793812
SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)C2CO2)NC(=O)[C@H]2CCCCN2CC1=O
InChI Key InChIKey=FBOIQKKEKYJGGI-UJYFFLPMSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50366693
Found 2 hits for monomerid = 50366693
Affinity DataIC50: <0.100nMAssay Description:Inhibitory activity against histone deacetylase (HDAC) derived from partially purified extracts of human HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against histone deacetylase (HDAC) from partially purified extracts of Eimeria tenella protozoal cellsMore data for this Ligand-Target Pair